Citation:
Zha Xiaosong. Reductive Dehalogenation of Bromoform by Iron Based Bimetallic Materials[J]. Chemistry,
;2020, 83(2): 172-178.

-
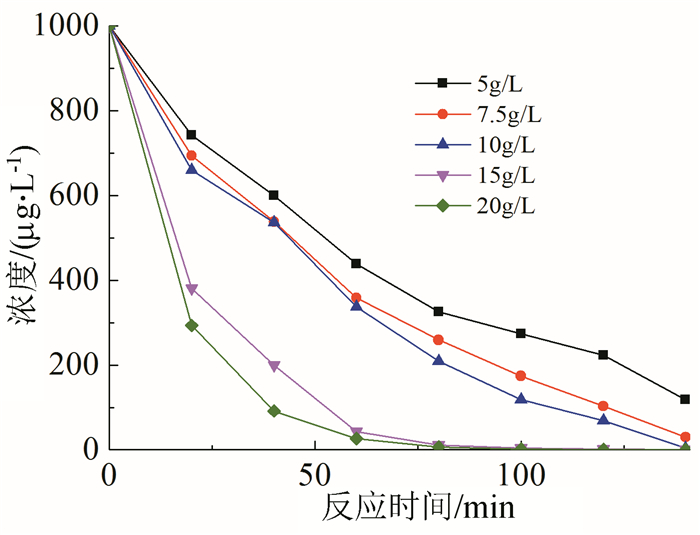
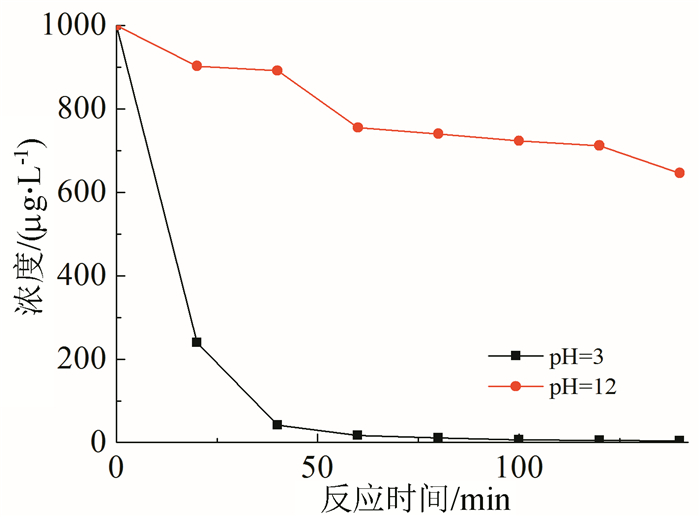
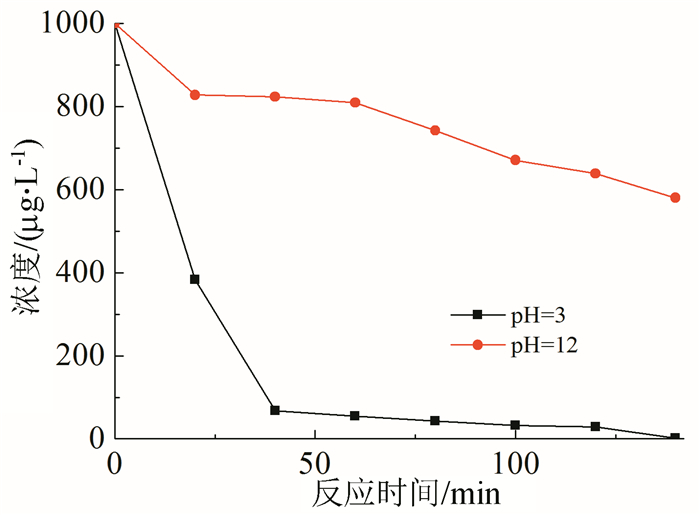
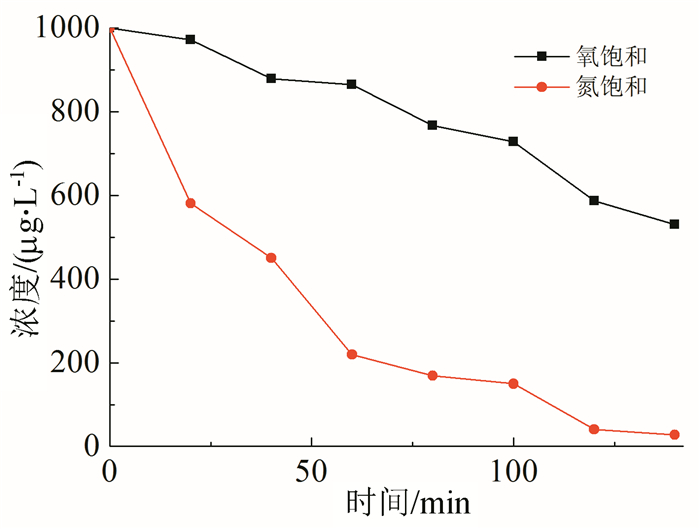
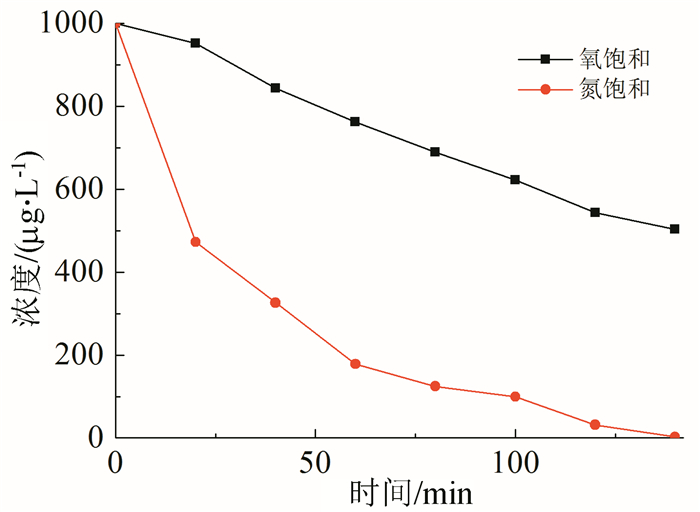
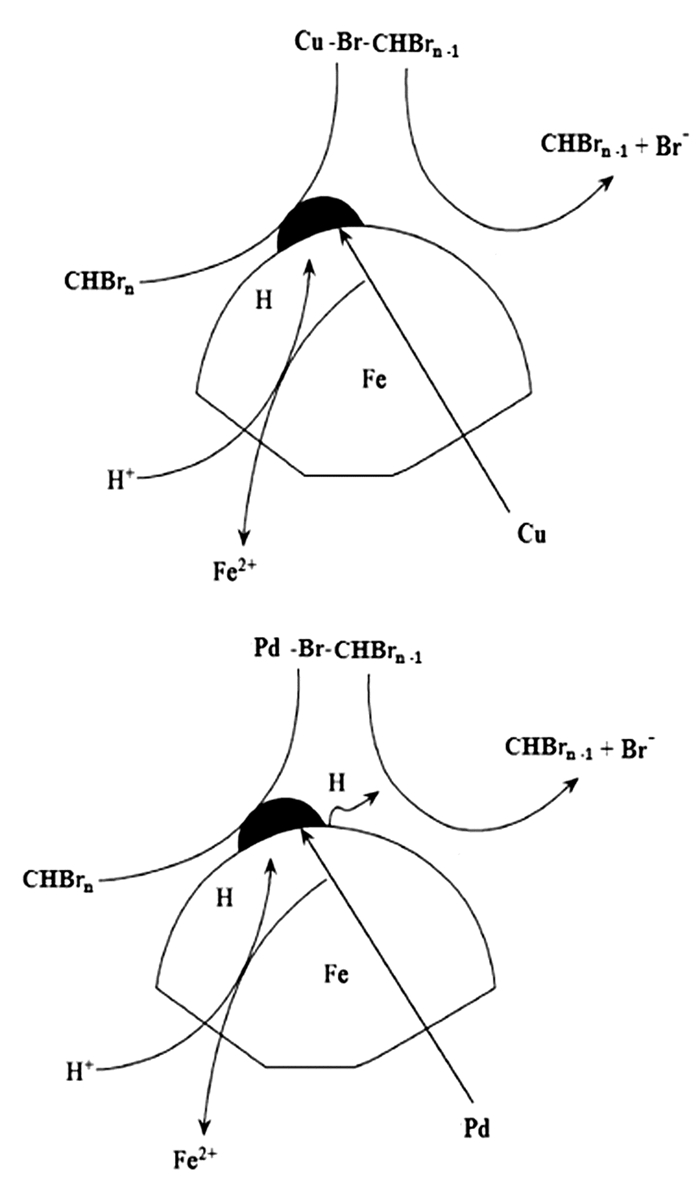
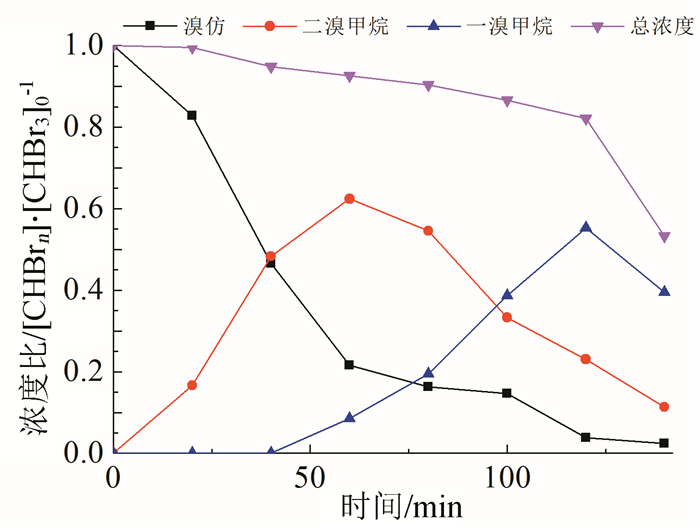
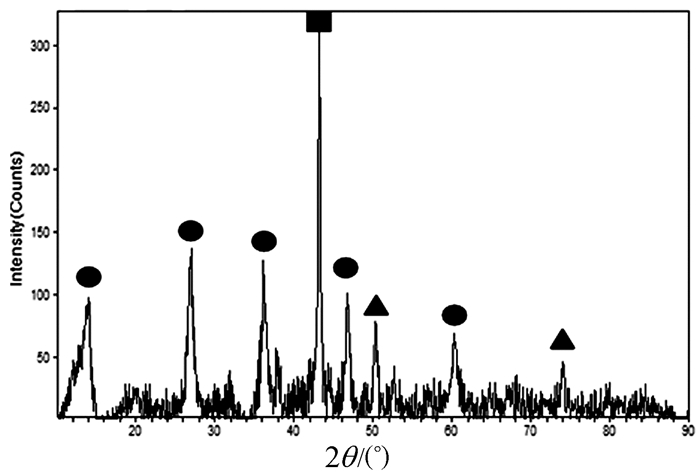
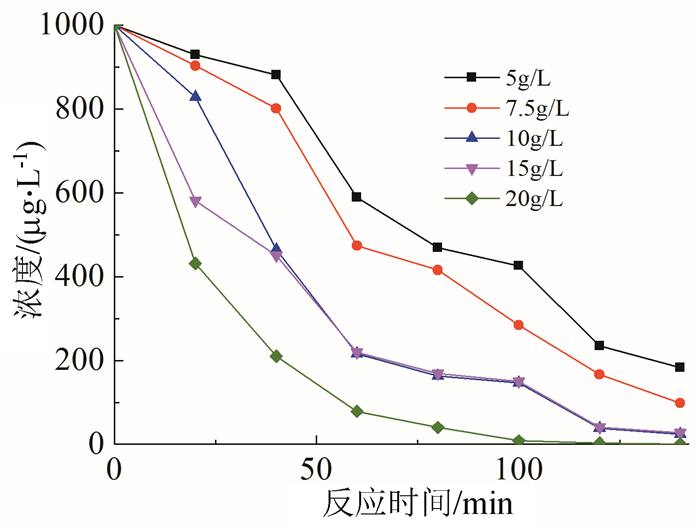
In this paper, iron based bimetallic materials (Cu/Fe and Pd/Fe) were prepared and their reductive dehalogenation effects on bromoform was investigated. The reduction effect of Cu/Fe and Pd/Fe bimetal materials on bromoform will increase as the addition amount of bimetal increases; the presence of high concentration of H+ in the solution is conducive to the reduction; furthermore, the presence of dissolved oxygen has a inhibitory effect on reductive dehalogenation. The reductive dehalogenation between bimetallic materials and bromoform includes direct reduction and indirect reduction. Bimetallic materials achieved high performance because galvanic cells were created between Fe (serving as an anode) and plating elements (serving as a cathode). This structure enhanced the reducibility of iron for reductive dehalogenation by facilitating iron corrosion. The Pd/Fe system showed a better performance than Cu/Fe, which was attributed to a higher potential gradient that promoting the hydrogen production.
-

-
-
[1]
Rook J J. Water Treat. Exam., 1974, 23: 234~243.
-
[2]
Wang X, Mao Y, Tang S, et al. Front. Environ. Sci. Eng., 2015, 9(1): 3~15.
-
[3]
Huang H, Zhu H H, Gan W H, et al. Chemosphere, 2017, 188: 257~264.
-
[4]
Kosyakov D M, Ul'yanovskii N V, Popov M S, et al. Water Res., 2017, 127: 183~190.
-
[5]
Sweeny K H. Reductive degradation treatment of industrial and municipal wastewaters//Proceedings of Water Reuse Symposium. AWWA, Research Foundation, Denver, Colo. 1979, 2: 1487~1497.
-
[6]
-
[7]
-
[8]
Pearson C R, Hozalski R M, Arnold W A. Environ. Toxicol. Chem., 2005, 24(12): 3037~3042.
-
[9]
Tang S, Wang X M, Yang H W, et al. Chemosphere, 2013, 90: 1563~1567.
-
[10]
Xie L, Shang C. Chemosphere, 2007, 66(9): 1652~1659.
-
[11]
-
[12]
Wang X Y, Ning P, Liu H L, et al. Appl. Catal. B, 2010, 94: 55~63.
-
[13]
Chen C, Wang X, Chang Y, et al. J. Environ. Sci., 2008, 20(8): 945~951.
-
[14]
Wang X Y, Chen C, Chang Y. J. Hazard. Mater., 2009, 161: 815~823.
-
[15]
Guasp E, Wei R. J. Chem. Technol. Biotechnol., 2003, 78: 654~658.
-
[16]
Lien H, Zhang W. J. Environ. Eng., 1999, 125(11): 1042~1047.
-
[17]
Cwiertny D M, Bransfield S J, Livi K J T, et al. Environ. Sci. Technol., 2006, 40: 6837~6843.
-
[18]
Hua G, Reckhow D A, Kim J. Environ. Sci. Technol., 2006, 40(9): 3050~3056.
-
[19]
American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 20th. Washington D C, USA: American Public Health Association/American Water Works Association/Water Environment Federation, 1998.
-
[20]
U.S. Environmental Protection Agency, 2003. Method 552.3: Determination of Haloacetic Acids and Dalapon in Drinking Water by Liquid-Liquid Microextraction, Derivatization, and Gas Chromatography with Electron Capture Detection. EPA 815-B-03-002. Revision 1.0.
-
[21]
Ghauch A, Assi H A, Bdeir S. J. Hazard. Mater., 2010, 182: 64~74.
-
[22]
Lien H L, Zhang W X. Appl. Catal. B, 2007, 77: 110~116.
- [23]
-
[1]
-

-
-
[1]
Guang Huang , Lei Li , Dingyi Zhang , Xingze Wang , Yugai Huang , Wenhui Liang , Zhifen Guo , Wenmei Jiao . Cobalt’s Valor, Nickel’s Foe: A Comprehensive Chemical Experiment Utilizing a Cobalt-based Imidazolate Framework for Nickel Ion Removal. University Chemistry, 2024, 39(8): 174-183. doi: 10.3866/PKU.DXHX202311051
-
[2]
Fugui XI , Du LI , Zhourui YAN , Hui WANG , Junyu XIANG , Zhiyun DONG . Functionalized zirconium metal-organic frameworks for the removal of tetracycline from water. Chinese Journal of Inorganic Chemistry, 2025, 41(4): 683-694. doi: 10.11862/CJIC.20240291
-
[3]
Jinyao Du , Xingchao Zang , Ningning Xu , Yongjun Liu , Weisi Guo . Electrochemical Thiocyanation of 4-Bromoethylbenzene. University Chemistry, 2024, 39(6): 312-317. doi: 10.3866/PKU.DXHX202310039
-
[4]
Bing WEI , Jianfan ZHANG , Zhe CHEN . Research progress in fine tuning of bimetallic nanocatalysts for electrocatalytic carbon dioxide reduction. Chinese Journal of Inorganic Chemistry, 2025, 41(3): 425-439. doi: 10.11862/CJIC.20240201
-
[5]
Zhuo Wang , Xue Bai , Kexin Zhang , Hongzhi Wang , Jiabao Dong , Yuan Gao , Bin Zhao . MOF-Templated Synthesis of Nitrogen-Doped Carbon for Enhanced Electrochemical Sodium Ion Storage and Removal. Acta Physico-Chimica Sinica, 2025, 41(3): 2405002-0. doi: 10.3866/PKU.WHXB202405002
-
[6]
Xiaoling LUO , Pintian ZOU , Xiaoyan WANG , Zheng LIU , Xiangfei KONG , Qun TANG , Sheng WANG . Synthesis, crystal structures, and properties of lanthanide metal-organic frameworks based on 2, 5-dibromoterephthalic acid ligand. Chinese Journal of Inorganic Chemistry, 2024, 40(6): 1143-1150. doi: 10.11862/CJIC.20230271
-
[7]
Jianding LI , Junyang FENG , Huimin REN , Gang LI . Proton conductive properties of a Hf(Ⅳ)-based metal-organic framework built by 2,5-dibromophenyl-4,6-dicarboxylic acid. Chinese Journal of Inorganic Chemistry, 2025, 41(6): 1094-1100. doi: 10.11862/CJIC.20240464
-
[8]
Yuanpei ZHANG , Jiahong WANG , Jinming HUANG , Zhi HU . Preparation of magnetic mesoporous carbon loaded nano zero-valent iron for removal of Cr(Ⅲ) organic complexes from high-salt wastewater. Chinese Journal of Inorganic Chemistry, 2024, 40(9): 1731-1742. doi: 10.11862/CJIC.20240077
-
[9]
Yuyao Wang , Zhitao Cao , Zeyu Du , Xinxin Cao , Shuquan Liang . Research Progress of Iron-based Polyanionic Cathode Materials for Sodium-Ion Batteries. Acta Physico-Chimica Sinica, 2025, 41(4): 2406014-0. doi: 10.3866/PKU.WHXB202406014
-
[10]
Xiangyu CAO , Jiaying ZHANG , Yun FENG , Linkun SHEN , Xiuling ZHANG , Juanzhi YAN . Synthesis and electrochemical properties of bimetallic-doped porous carbon cathode material. Chinese Journal of Inorganic Chemistry, 2025, 41(3): 509-520. doi: 10.11862/CJIC.20240270
-
[11]
Peng XU , Shasha WANG , Nannan CHEN , Ao WANG , Dongmei YU . Preparation of three-layer magnetic composite Fe3O4@polyacrylic acid@ZiF-8 for efficient removal of malachite green in water. Chinese Journal of Inorganic Chemistry, 2024, 40(3): 544-554. doi: 10.11862/CJIC.20230239
-
[12]
Hui-Ying Chen , Hao-Lin Zhu , Pei-Qin Liao , Xiao-Ming Chen . Integration of Ru(Ⅱ)-Bipyridyl and Zinc(Ⅱ)-Porphyrin Moieties in a Metal-Organic Framework for Efficient Overall CO2 Photoreduction. Acta Physico-Chimica Sinica, 2024, 40(4): 2306046-0. doi: 10.3866/PKU.WHXB202306046
-
[13]
Zhicheng JU , Wenxuan FU , Baoyan WANG , Ao LUO , Jiangmin JIANG , Yueli SHI , Yongli CUI . MOF-derived nickel-cobalt bimetallic sulfide microspheres coated by carbon: Preparation and long cycling performance for sodium storage. Chinese Journal of Inorganic Chemistry, 2025, 41(4): 661-674. doi: 10.11862/CJIC.20240363
-
[14]
Xinlong XU , Chunxue JING , Yuzhen CHEN . Bimetallic MOF-74 and derivatives: Fabrication and efficient electrocatalytic biomass conversion. Chinese Journal of Inorganic Chemistry, 2025, 41(8): 1545-1554. doi: 10.11862/CJIC.20250046
-
[15]
Shiqian WEI , Xinyu TIAN , Hong LIU , Maoxia CHEN , Fan TANG , Qiang FAN , Weifeng FAN , Yu HU . Oxygen reduction reaction/oxygen evolution reaction catalytic performances of different active sites on nitrogen-doped graphene loaded with iron single atoms. Chinese Journal of Inorganic Chemistry, 2025, 41(9): 1776-1788. doi: 10.11862/CJIC.20250102
-
[16]
Fangfang WANG , Jiaqi CHEN , Weiyin SUN . CuBi@Cu-MOF composite catalysts for electrocatalytic CO2 reduction to HCOOH. Chinese Journal of Inorganic Chemistry, 2025, 41(1): 97-104. doi: 10.11862/CJIC.20240350
-
[17]
Runhua Chen , Qiong Wu , Jingchen Luo , Xiaolong Zu , Shan Zhu , Yongfu Sun . Defective Ultrathin Two-Dimensional Materials for Photo-/Electrocatalytic CO2 Reduction: Fundamentals and Perspectives. Acta Physico-Chimica Sinica, 2025, 41(3): 2308052-0. doi: 10.3866/PKU.WHXB202308052
-
[18]
Qiang Zhang , Yuanbiao Huang , Rong Cao . Imidazolium-Based Materials for CO2 Electroreduction. Acta Physico-Chimica Sinica, 2024, 40(4): 2306040-0. doi: 10.3866/PKU.WHXB202306040
-
[19]
Zilin Hu , Yaoshen Niu , Xiaohui Rong , Yongsheng Hu . Suppression of Voltage Decay through Ni3+ Barrier in Anionic-Redox Active Cathode for Na-Ion Batteries. Acta Physico-Chimica Sinica, 2024, 40(6): 2306005-0. doi: 10.3866/PKU.WHXB202306005
-
[20]
Xiaoyang Li , Xiaowei Huang , Yimeng Zhang , Huan Liu , Shao Jin , Junpeng Zhuang . Comprehensive Chemical Experiments on the Synthesis of 1,3-Dibromo-5,5-Dimethylhydantoin and Its Application as a Brominating Reagent. University Chemistry, 2025, 40(7): 286-293. doi: 10.12461/PKU.DXHX202408035
-
[1]
Metrics
- PDF Downloads(9)
- Abstract views(1016)
- HTML views(334)

 Login In
Login In




 DownLoad:
DownLoad: