Citation:
Wang Kaixuan, Wang Lanzhi. Unexpected Rearrangement Reaction and Synthesis of Benzoxazoles[J]. Chinese Journal of Organic Chemistry,
;2019, 39(4): 1147-1152.
doi:
10.6023/cjoc201809038

-
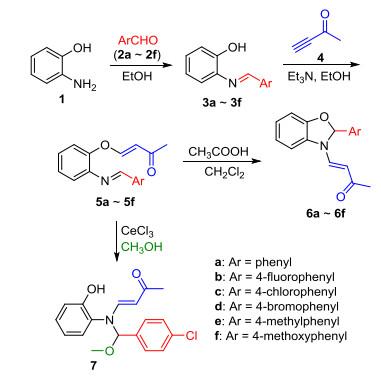
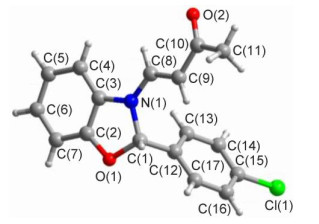
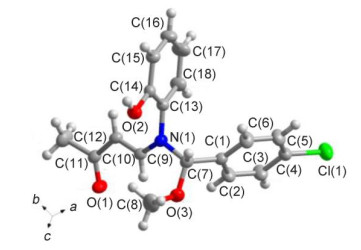
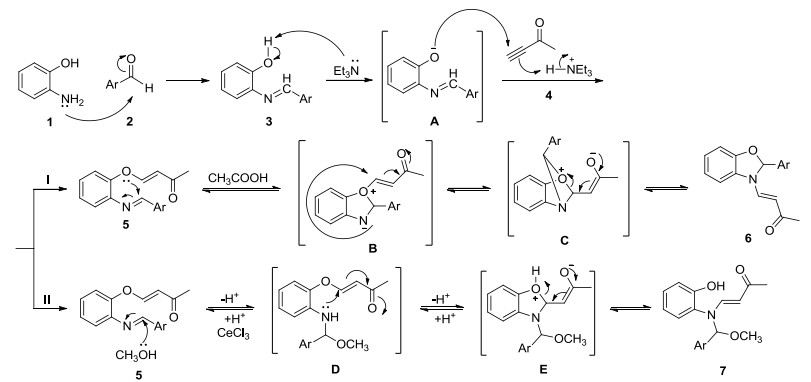
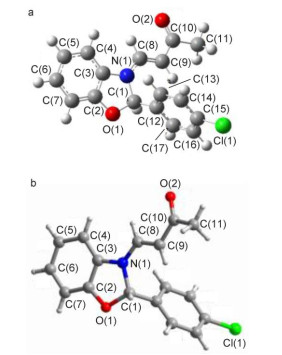
Novel series of rearrangement reactions were herein reported that enable access to a variety of unique 2-aryl-3-(3'-oxobutenyl)-benzoxazole compounds 6a~6f from 2-aminophenol, aromatic aldehyde and 3-butyn-2-one as materials by nucleophilic conjugate addition, dehydration and rearrangement reactions and intramolecular cyclization in the presence of a catalytic amount of CH3COOH in CH2Cl2 at ambient temperature. On the basis of products and intermediate products, a series of possible mechanism was presented and theoretically verified by density functional theory (DFT) method at B3LYP/6-31G (d, p) level from both molecular energy and atomic charge in Gaussian 03 package. The results show that the theory and experiment consistently explain the rationality of the reaction mechanism. The mechanism of rearrangement provides a basis for further study of this type of reaction. The advantage of this method is that a novel structure of benzoxazole derivative was synthesized successfully via a series of rearrangement reactions. Therefore, this method can be used as an attractive strategy for practical synthesis of nitrogen heterocyclic compounds.
-

-
-
[1]
Zhou, W. J.; Zhang, L.; Xiao, W.; Chen, H. J.; Wu, W. N.; Ouyang, G. P. J. Heterocycl. Chem. 2017, 54, 1423. doi: 10.1002/jhet.v54.2
-
[2]
Praveen, C.; Nandakumar, A.; Dheenkumar, P.; Muralidharan, D.; P. Perumal, P. T. J. Chem. Sci. 2012, 124, 609. doi: 10.1007/s12039-012-0251-3
-
[3]
Temiz-Arpaci, O.; Arisoy, M.; Sac, D.; Doganc, F.; Tasci, M.; Senol, F. S.; Orhan, I. E. Z. Naturforsch., C 2016, 71, 409. doi: 10.1515/znc-2016-0087
-
[4]
Reen, G. K, ; Kumar, A.; Sharma, P. Med. Chem. Res. 2017, 26, 3336. doi: 10.1007/s00044-017-2026-3
-
[5]
Yildiz-Oren, I.; Yalcin, I.; Aki-Sener, E.; Ucarturk, N. Eur. J. Med. Chem. 2004, 9, 291.
-
[6]
Tasci, M.; Temiz-Arpaci, O.; Kaynak-Onurdag, F.; Okten, S. Indian J. Chem., Sect. B:Org. Chem. Incl. Med. Chem. 2018, 57, 385.
-
[7]
Tseng, C.-H.; Lin, C.-K.; Chen, Y.-L.; Tseng, C.-K.; Lee, J.-Y. Lee, J.-C. Eur. J. Med. Chem. 2018, 143, 970. doi: 10.1016/j.ejmech.2017.12.006
-
[8]
Henderson, J. A.; Bilimoria, D.; Bubenik, M.; Cadilhac, C.; Cottrell, K. M.; Denis, F.; Dietrich, E.; Ewing, N.; Falardeau, G.; Giroux, S.; L'Heureux, L.; Liu, B.; Mani, N.; Morris, M.; Nicolas, O.; Pereira, O. Z.; Poisson, C.; Reddy, T. J.; Selliah, S.; Shawgo, R. S.; Vaillancourt, L.; Wang, J.; Xu, J.; Chauret, N.; Berlioz-Seux, F.; Chan, L. C.; Das, S. K.; Grillot, A.-L.; Bennani, Y. L.; Maxwell, J. P. Bioorg. Med. Chem. Lett. 2015, 25, 948. doi: 10.1016/j.bmcl.2014.12.042
-
[9]
Zilifdar, F.; Foto, E.; Ertan-Bolelli, T.; Aki-Yalcin, E.; Yalcin, I.; Diril, N. Arch. Pharm. 2018, 351.
-
[10]
Khajondetchairit, P.; Phuangsawai, O.; Suphakun, P.; Rattanabunyong, S.; Choowongkomon, K.; Gleeson, M. P. Chem. Biol. Drug Des. 2017, 90, 987. doi: 10.1111/cbdd.2017.90.issue-5
-
[11]
Goekhan-Kelekci, N.; Koeksal, M.; Uenuevar, S.; Aktay, G.; Erdogan, H. J. Enzyme Inhib. Med. Chem. 2009, 24, 29. doi: 10.1080/14756360701841772
-
[12]
Eren, G.; Unlu, S.; Nunez, M. T.; Labeaga, L.; Ledo, F.; Entrena, A.; Banoglu, E.; Costantino, G.; Sahin, M. F. Bioorg. Med. Chem. 2010, 18, 6367. doi: 10.1016/j.bmc.2010.07.009
-
[13]
Jayanna, N. D.; Vagdevi, H. M.; Dharshan, J. C.; Raghavendra, R. Telkar, S. B. Med. Chem. Res. 2013, 22, 5814. doi: 10.1007/s00044-013-0565-9
-
[14]
Wei, P.-F.; Qi, M.-Z.; Wang, Z.-P.; Ding, S.-Y.; Yu, W.; Liu, Q.; Wang, L.-K.; Wang, H.-Z.; An, W.-K.; Wang, W. J. Am. Chem. Soc. 2018, 140, 4623. doi: 10.1021/jacs.8b00571
-
[15]
Yeh, V. S. C. Tetrahedron 2004, 60, 11995. doi: 10.1016/j.tet.2004.10.001
-
[16]
Leaver, I. H.; Milligan, B. Dyes Pigm. 1984, 5, 109. doi: 10.1016/0143-7208(84)80008-X
-
[17]
Chen, T.-R. J. Organomet. Chem. 2008, 693, 3117. doi: 10.1016/j.jorganchem.2008.06.034
-
[18]
Dunwell, D. W.; Evans, D. Hicks, T. A. J. Med. Chem. 1975, 18, 1158. doi: 10.1021/jm00245a026
-
[19]
Grossi, G.; Di Braccio, M.; Roma, G.; Ballabeni, V.; Tognolini, M.; Calcina, F.; Barocelli, E. Eur. J. Med. Chem. 2002, 37, 933 doi: 10.1016/S0223-5234(02)01400-9
-
[20]
Smith, R. H., Jr.; Jorgensen, W. L.; Tirado-Rives, J. Lamb, M. L.; Janssen, P. A.; Michejda, C. J.; Kroeger Smith, M. B. J. Med. Chem. 1998, 41, 5272 doi: 10.1021/jm9804174
-
[21]
Liu, D.; Chen, H. Y.; Zhang, J. Y.; Huang, J. Y.; Li, Y. M.; Peng, Q. M. Appl. Surf. Sci. 2018, 456, 59. doi: 10.1016/j.apsusc.2018.06.123
-
[22]
Li, X.-Q. Li; Wang, L.-Z. Chin. Chem. Lett. 2014, 25, 327. doi: 10.1016/j.cclet.2013.11.035
-
[23]
Yin, L.-Y.; Wang, L.-Z. Tetrahedron Lett. 2016, 57, 5935. doi: 10.1016/j.tetlet.2016.11.089
-
[24]
Qiu, Z.-L.; Wang, L.-Z.; Li, W.-H.; Li. Y. Acta Chim. Sinica 2011, 69, 1217(in Chinese).
-
[1]
-

-
-
[1]
Tongyan Yu , Pan Xu . Visible-Light Photocatalyzed Radical Rearrangement Reaction. University Chemistry, 2025, 40(7): 169-176. doi: 10.12461/PKU.DXHX202409070
-
[2]
Xinxin Wu . 基础有机化学教学中自由基重排反应的课程设计及其课程思政元素的融入. University Chemistry, 2025, 40(6): 316-325. doi: 10.12461/PKU.DXHX202408055
-
[3]
Yuting Zhang , Zhiqian Wang . Methods and Case Studies for In-Depth Learning of the Aldol Reaction Based on Its Reversible Nature. University Chemistry, 2024, 39(7): 377-380. doi: 10.3866/PKU.DXHX202311037
-
[4]
Ronghao Zhao , Yifan Liang , Mengyao Shi , Rongxiu Zhu , Dongju Zhang . Investigation into the Mechanism and Migratory Aptitude of Typical Pinacol Rearrangement Reactions: A Research-Oriented Computational Chemistry Experiment. University Chemistry, 2024, 39(4): 305-313. doi: 10.3866/PKU.DXHX202309101
-
[5]
Jiajie Li , Xiaocong Ma , Jufang Zheng , Qiang Wan , Xiaoshun Zhou , Yahao Wang . Recent Advances in In-Situ Raman Spectroscopy for Investigating Electrocatalytic Organic Reaction Mechanisms. University Chemistry, 2025, 40(4): 261-276. doi: 10.12461/PKU.DXHX202406117
-
[6]
Lixing ZHANG , Yaowen WANG , Xu HAN , Junhong ZHOU , Jinghui WANG , Liping LI , Guangshe LI . Research progress in the synthesis of fluorine-containing perovskites and their derivatives. Chinese Journal of Inorganic Chemistry, 2025, 41(9): 1689-1701. doi: 10.11862/CJIC.20250007
-
[7]
Bin SUN , Heyan JIANG . Glucose-modified bis-Schiff bases: Synthesis and bio-activities in Alzheimer′s disease therapy. Chinese Journal of Inorganic Chemistry, 2025, 41(7): 1338-1350. doi: 10.11862/CJIC.20240428
-
[8]
Xinyi Zhang , Kai Ren , Yanning Liu , Zhenyi Gu , Zhixiong Huang , Shuohang Zheng , Xiaotong Wang , Jinzhi Guo , Igor V. Zatovsky , Junming Cao , Xinglong Wu . Progress on Entropy Production Engineering for Electrochemical Catalysis. Acta Physico-Chimica Sinica, 2024, 40(7): 2307057-0. doi: 10.3866/PKU.WHXB202307057
-
[9]
Wentao Lin , Wenfeng Wang , Yaofeng Yuan , Chunfa Xu . Concerted Nucleophilic Aromatic Substitution Reactions. University Chemistry, 2024, 39(6): 226-230. doi: 10.3866/PKU.DXHX202310095
-
[10]
Zhi Chai , Huashan Huang , Xukai Shi , Yujing Lan , Zhentao Yuan , Hong Yan . Wittig反应的立体选择性. University Chemistry, 2025, 40(8): 192-201. doi: 10.12461/PKU.DXHX202410046
-
[11]
Bolin Sun , Jie Chen , Ling Zhou . 乙烯型卤代烃的亲核取代反应. University Chemistry, 2025, 40(8): 152-157. doi: 10.12461/PKU.DXHX202410032
-
[12]
Hongting Yan , Aili Feng , Rongxiu Zhu , Lei Liu , Dongju Zhang . Reexamination of the Iodine-Catalyzed Chlorination Reaction of Chlorobenzene Using Computational Chemistry Methods. University Chemistry, 2025, 40(3): 16-22. doi: 10.12461/PKU.DXHX202403010
-
[13]
Aili Feng , Xin Lu , Peng Liu , Dongju Zhang . Computational Chemistry Study of Acid-Catalyzed Esterification Reactions between Carboxylic Acids and Alcohols. University Chemistry, 2025, 40(3): 92-99. doi: 10.12461/PKU.DXHX202405072
-
[14]
Guowen Xing , Guangjian Liu , Le Chang . Five Types of Reactions of Carbonyl Oxonium Intermediates in University Organic Chemistry Teaching. University Chemistry, 2025, 40(4): 282-290. doi: 10.12461/PKU.DXHX202407058
-
[15]
Ling Fan , Meili Pang , Yeyun Zhang , Yanmei Wang , Zhenfeng Shang . Quantum Chemistry Calculation Research on the Diels-Alder Reaction of Anthracene and Maleic Anhydride: Introduction to a Computational Chemistry Experiment. University Chemistry, 2024, 39(4): 133-139. doi: 10.3866/PKU.DXHX202309024
-
[16]
Jiabo Huang , Quanxin Li , Zhongyan Cao , Li Dang , Shaofei Ni . Elucidating the Mechanism of Beckmann Rearrangement Reaction Using Quantum Chemical Calculations. University Chemistry, 2025, 40(3): 153-159. doi: 10.12461/PKU.DXHX202405172
-
[17]
Jing WU , Puzhen HUI , Huilin ZHENG , Pingchuan YUAN , Chunfei WANG , Hui WANG , Xiaoxia GU . Synthesis, crystal structures, and antitumor activities of transition metal complexes incorporating a naphthol-aldehyde Schiff base ligand. Chinese Journal of Inorganic Chemistry, 2024, 40(12): 2422-2428. doi: 10.11862/CJIC.20240278
-
[18]
Ying Chen , Ronghua Yan , Weiyan Yin . Research Progress on the Synthesis of Metal Single-Atom Catalysts and Their Applications in Electrocatalytic Hydrogen Evolution Reactions. University Chemistry, 2025, 40(9): 344-353. doi: 10.12461/PKU.DXHX202503066
-
[19]
Xinting XIONG , Zhiqiang XIONG , Panlei XIAO , Xuliang NIE , Xiuying SONG , Xiuguang YI . Synthesis, crystal structures, Hirshfeld surface analysis, and antifungal activity of two complexes Na(Ⅰ)/Cd(Ⅱ) assembled by 5-bromo-2-hydroxybenzoic acid ligands. Chinese Journal of Inorganic Chemistry, 2024, 40(9): 1661-1670. doi: 10.11862/CJIC.20240145
-
[20]
Lifang HE , Wenjie TANG , Yaoze LUO , Mingsheng LIANG , Jianxin TANG , Yuxuan WU , Fuxing ZHANG , Xiaoming ZHU . Synthesis, structure, and anticancer activity of two dialkyltin complexes constructed based on 2, 2′-bipyridin-6, 6′-dicarboxylic acid. Chinese Journal of Inorganic Chemistry, 2025, 41(8): 1601-1609. doi: 10.11862/CJIC.20250012
-
[1]
Metrics
- PDF Downloads(11)
- Abstract views(1068)
- HTML views(110)

 Login In
Login In




 DownLoad:
DownLoad: