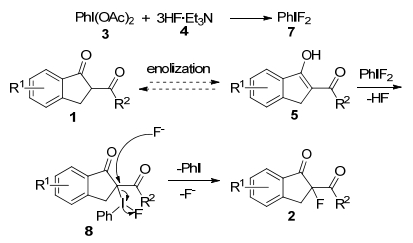
-
[1]
(a) Isanbor, C.; Hagan, D. O. J. Fluorine Chem. 2006, 127, 303.
(b) Kirk, K. L. J. Fluorine Chem. 2006, 127, 1013.
(c) Purser, S.; Moore, P. R.; Swallow, S.; Gouvemeur, V. Chem. Soc. Rev. 2008, 37, 320.
(d) Jiang, W.; Sanchez-Rosello, M.; Acena, J. L.; Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Hong, L. Chem. Rev. 2014, 114, 2432.
(e) Xu, J. X.; Hu, Y. L.; Huang, D. F.; Wang, K. H.; Xu, C. M.; Niu, T. Adv. Synth. Catal. 2012, 354, 515.
(f) Koike, T.; Akita, M. J. Fluorine Chem. 2014, 167, 30.
(g) Wang, X.; Liu, G. K.; Xu, X. H.; Naoyuki, S.; Etsuko, T.; Norio, S. Angew. Chem., Int. Ed. 2014, 53, 1827.
(h) Sugiishi, K.; Matsugi, M.; Hamamoto, H.; Amii, H. RSC. Adv. 2015, 5, 17269.
(i) Köckinger, M.; Ciaglia, T.; Bersier, M.; Hanselmann, P.; Gutmann, B.; Kappe, C. O. Green Chem. 2018, 20, 108.
-
[2]
(a) Zhao, Y. M.; Cheung, M. S.; Lin, Z. Y.; Sun, J. W. Angew. Chem., Int. Ed. 2012, 51, 10359.
(b) Suzuki, S.; Kitamura, Y.; Lectard, S.; Hamashima, Y.; Sodeoka, M. Angew. Chem., Int. Ed. 2012, 51, 4581.
(c) Shibatomi, K.; Soga, Y.; Narayama, A.; Fujisawa, I.; Iwasa, S. J. Am. Chem. Soc. 2012, 134, 9836.
(d) Ma, B. W.; Lin, X. B.; Lin, L. L.; Feng, X. M.; Liu, X. H. J. Org. Chem. 2017, 82, 701.
(e) Salomon, P.; Zard, S. Z. Org. Lett. 2014, 16, 1482.
(f) Qi, X. X.; Yu, F.; Chen, P. H.; Liu, G. S. Angew. Chem., Int. Ed. 2017, 56, 12692.
(g) Molnár, I. G.; Gilmour, R. J. Am. Chem. Soc. 2016, 138, 5004.
(h) Ma, X. H.; Diane, M.; Ralph, G.; Chen, C.; Biscoe, M. R. Angew. Chem., Int. Ed. 2017, 56, 12663.
(i) Liang, T.; Neumann, C. N.; Ritter, T. Angew. Chem., Int. Ed. 2013, 52, 8214.
-
[3]
(a) Shibatomi, K.; Tsuzuki, Y.; Iwasa, S. Chem. Lett. 2008, 37, 1098.
(b) Shibatomi, K.; Kitahara, K.; Sasaki, N.; Kawasaki, Y.; Fujisawa, I.; Iwasa, S. Nat. Commun. 2017, 8, 15600.
(c) Prusov, E. V. Angew. Chem., Int. Ed. 2017, 56, 14356.
(d) Kang, S. H.; Kim, D. Y. Adv. Synth. Catal. 2010, 352, 2783.
(e) Kitamura, T.; Kuriki, S.; Morshed, M. H.; Hori, Y. Org. Lett. 2011, 13, 2392.
(f) Kitamura, T.; Muta, K.; Muta, K. J. Org. Chem. 2014, 79, 5842.
-
[4]
(a) Li, C. K.; Breit, B. J. Am. Chem. Soc. 2014, 136, 862.
(b) Turnbull, B. W. H.; Evans, P. A. J. Am. Chem. Soc. 2015, 137, 6156.
(c) Roy, S. R.; Didier, D.; Kleiner, A.; Marek, I. Chem. Sci. 2016, 7, 5989.
(d) Starkov, P.; Moore, J. T.; Duquette, D. C.; Stoltz, B. M.; Marek, I. J. Am. Chem. Soc. 2017, 139, 9615.
(e) Liu, J. Y.; Zhao, J.; Zhang, J. L.; Xu, P. F. Org. Lett. 2017, 19, 1846.
-
[5]
(a) Gu, X.; Zhang, Y.; Xu, Z. J.; Che, C. M. Chem. Commun. 2014, 50, 7870.
(b) Wang, Y. F.; Wang, H. J.; Jiang, Y. D.; Zhang, C.; Shao, J. J.; Xu, D. Q. Green. Chem. 2017, 19, 1674.
(c) Zheng, L. S.; Wei, Y. L.; Jiang, K. Z.; Deng, Y.; Zheng, Z. J.; Xu, L. W. Adv. Synth. Catal. 2014, 356, 3769.
(d) Kwon, S. J.; Kim, D. Y. J. Fluorine Chem. 2015, 180, 201.
-
[6]
Hintermann, L.; Togni, A. T. Angew. Chem., Int. Ed. 2000, 39, 4359.
doi: 10.1002/(ISSN)1521-3773
-
[7]
Wang, X. S.; Lan, Q.; Shirakawa, S.; Maruoka, K. Chem. Commun. 2010, 46, 321.
doi: 10.1039/B920099A
-
[8]
(a) Nash, T. J.; Pattison, G. Eur. J. Org. Chem. 2015, 3779.
(b) Suzuki, S.; Kamo, T.; Fukushi, K.; Hiramatsu, T.; Tokunaga, E.; Dohi, T.; Kita, Y.; Shibata, N. Chem. Sci. 2014, 5, 2754.
(c) Pluta, R.; Krach, P. E.; Cavallo, L.; Falvienne, L.; Rueping, M. ACS Catal. 2018, 8, 2582.
(d) Wang, Y.; Yuan, H.; Lu, H. F.; Zheng, W. H. Org. Lett. 2018, 20, 2555.
(e) Goldbberg, N. W.; Shen, X.; Li, J. K.; Ritter, T. Org. Lett. 2016, 18, 6102.
(f) Woerly, E. M.; Banik, S. M.; Jacobsen, E. N. J. Am. Chem. Soc. 2016, 138, 13858.
(g) Satoshi, M.; Hiroki, O.; Ayako, K.; Kanami, K.; Yuki, M.; Jun, I.; Kodai, N.; Ryo, H.; Toshiya, U.; Kenya, C. Org. Lett. 2017, 19, 2572.
-
[9]
Gondo, K; Kitamura, T. Molecules 2012, 17, 6625.
doi: 10.3390/molecules17066625
-
[10]
(a) Kitamura, T.; Muta, K.; Muta, K. J. Org. Chem. 2014, 79, 5842.
(b) Wirth, T. Angew. Chem., Int. Ed. 2005, 44, 3656.
(c)Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328.
(d) Zhang, B. B.; Zhang, X.; Hu, B.; Sun, D. S.; Wang, S. L.; Zhang-Negrerie, D.; Du, Y. F. Org. Lett. 2017, 19, 902.
-
[11]
Uyanik, M.; Yasui, T.; Ishihara, K. J. Org. Chem. 2017, 82, 11946.
doi: 10.1021/acs.joc.7b01941
-
[12]
Banik, S. M.; Medley, J. W.; Jacobsen, E. N. J. Am. Chem. Soc. 2018, 138, 5000.
-
[13]
Kitamura, T.; Muta, K.; Oyamada, J. J. Org. Chem. 2015, 80, 10431.
doi: 10.1021/acs.joc.5b01929

 Login In
Login In

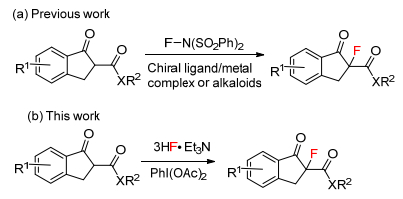





 DownLoad:
DownLoad: