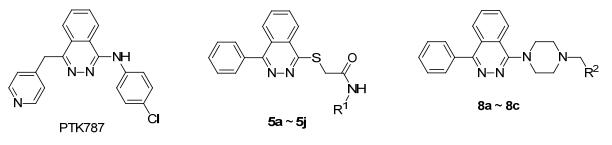
 Figure1.
Structures of PTK787 and the target compounds
Figure1.
Structures of PTK787 and the target compounds

1-苯基-4-取代酞嗪衍生物合成及抗肿瘤活性评价
English
Synthesis and Antitumor Activity of 1-Phenyl-4-substituted Phthalazine Derivatives
-
Key words:
- synthesis
- / phthalazine derivative
- / antitumor activity
-
1 Introduction
According to the statistics of World Health Organization in the world, there are 14 million new cases of cancer in 2012. The number of new cases is expected to increase by about 70% over the next two decades, and there are 8.8 million deaths in 2015, nearly one in six died of cancer in the word, nearly 70% of cancer deaths occur in developing countries. At present, many chemotherapy drugs have been developed for the treatment of various cancers, but there are large side effects. Therefore, it is urgent to find new molecules for the treatment of cancer.[1] Phthalazine is an important nitrogen containing hetero cyclic compound, which has a variety of pharmacological activity. Phthalazine-based compounds exhibit various pharmacological activities such as anti-cancer, anti-diabetic, anti-asthma, anti-histamines, antihypertensive, anti-thrombotic, anti-inflammatory, analgesic, anti-depressant and anti-microbial, which make it a basic scaffold for the design and development of new drugs.[2~6] Studies have shown that 1, 4-substituted phthalazine derivatives have an excellent antitumor activity, and PTK787 is one of the most excellent compounds. PTK787 is a VEGFR-2 inhibitor and it is currently used in clinical phase Ⅲ for the treatment of rectal cancer, prostate cancer, and advanced colorectal cancer.[6~11] We aimed to develop a potent compound for the treatment of cancer on the basis of the structure of PTK787 (Figure 1), and we also found a compound with a better antitumor activity in our early work.[12] In this work, we replaced methylidene pyridine group with phenyl group to increase the lipophilic and stereo regular effect of the compounds. In order to increase the length and hydrophilicity of the compounds, a series of 1-phenyl-4-thiop-thalazine and 1-phenyl-4-piperazinylphthalazine compounds were synthesized (Figure 1). In addition, the cytotoxicities of synthetic compounds against four human cancer cell lines in vitro were evaluated. The synthetic route of the target compounds is shown in Scheme 1.
2 Results and discussion
2.1 Chemistry
Compound 2 was synthesized from 2-benzoyl-benzoic acid and hydrazine hydrate according to the reportedprocedures, [13~18] compound 3 was obtained from the reaction of compound 2 with phosphorus pentasulfide. Compound 3 reacted with 2-chloro-N-(substituted phenyl)-acetamide (4) to afford compounds 5a~5j via nucleophilic substitution reaction, and water and ethanol were used as a mixed solvent.
Compound 6 was synthesized by the reaction of compound 2 with phosphorus oxychloride, compound 7 was synthesized from the reaction of compound 6 with piperazine, and compound 7 reacted with chloromethyl compounds to afford compounds 8a~8c.
2.2 Cytotoxic activity
All synthesized compounds were tested for their cytotoxic activity against four cancer cell lines. It can be seen that compound 8c has a high potency against four human cancer cell lines (Table 1). The IC50 value of 8c ranges from 6.31 to 20.13 μmol•L-1 against MGC-803, EC-109, PC-3 and SMMC-7721, and it displayed comparable or better IC50 values (6.31 μmol•L-1 against EC-109 and 16.72 μmol•L-1 against SMMC-7721) to that of 5-fluorouracil (5-Fu) (14.37 μmol•L-1 against EC-109 and 16.72 μmol•L-1 against SMMC-7721). In addition, compound 5f (8.13 μmol•L-1 against EC-109) also showed better activity than 5-Fu (14.37 μmol•L-1 against EC-109). Among the compounds 5a~5j, 5a and 5b have less cytotoxicity than 5f, indicating that it may be helpful for the antitumor activity when p-position has an electron-donating group. Compared 5c and 5j, it can be found that when o-position and p-position are substituted by electron-donating groups, o-position may be have a better antitumor activity. Compared 5d and 5e, it can be found that when o-position and p-position have two electronwithdrawing atoms, p-position may be helpful for the antitumor activity. 5i has a poor antitumor activity, indicating that a strong conjugated system has less influence on antitumor activity. Among all synthesized compounds, 8a~8c showed better activity than 5a~5j, indicating that piperazine unit may be helpful for antitumor activity.
Compound MGC-803 EC-109 PC-3 SMMC-7721 5a 26.43±0.83 40.36±3.20 36.04±1.83 30.67±2.77 5b 32.67±1.47 > 64 > 64 > 64 5c > 64 > 64 49.17±2.96 > 64 5d > 64 58.85±3.67 32.34±1.38 > 64 5e 21.90±1.42 > 64 > 64 44.76±3.46 5f 20.37±2.48 8.13±0.68 28.67±2.47 > 64 5g > 64 > 64 > 64 > 64 5h > 64 > 64 > 64 > 64 5i 50.45±2.83 42.48±0.38 > 64 > 64 5j > 64 40.39±2.84 > 64 > 64 8a > 64 > 64 36.04±1.83 30.67±2.77 8b 32.67±1.47 20.26±0.39 37.96±1.23 42.28±1.71 8c 12.34±0.67 6.31±0.82 20.13±1.06 16.72±0.38 5-Fu 8.41±0.32 14.37±0.29 7.15±0.81 15.79±0.34 a Antitumor activity was assayed by exposure for 72 h to substances and expressed as concentration required to inhibit tumor cell proliferation by 50% (IC50). Dates are presented as the means±SDs of three independent experiments. 3 Conclusions
A series of 1-phenyl-4-substituted phthalazine derivatives were synthesized. The structures of these compounds were confirmed by 1H NMR, 13C NMR and HRMS spectroscopy. The anti-tumor activity of the synthesized compounds were tested by MTT assay. MGC-803 (Human gastric cancer cells), EC-9706 (Human esophageal cancer cells), PC-3 (Human prostate cancer cells), and SMMC-7721 (Human hepatocytes) were tested in order to evaluate the cytotoxicity of the synthesized compounds, and 5-Fu was used as a positive control drug. Among them, compound 8c (IC50 values of 12.34, 6.31, 20.13, 16.72 μmol•L-1 against MGC-803, EC-9706, PC-3 and SMMC-7721, respectively) is the most effective in inhibiting cell proliferation in four human cancer cell lines, and the cytotoxicity to EC-109 and SMMC-7721 is superior or close to 5-fuorouracil. Among the target compounds, the selectivity of compound 5f (IC50 value of 8.13 μmol•L-1 against EC-109) was the best, and it had no obvious toxicity against the SMMC-7721 cell line. In view of the evaluation of the antitumor activity in vitro, the cytotoxicity of the synthesized compounds is in the range below.
4 Experimental
4.1 Materials
Silica gel: China Qingdao Ocean Chemical Group Corporation. Column chromatography silica gel: Shanghai May Fourth Chemical Reagent Factory. Acetyl chloride: Tianjin Miou Chemical Reagent Co., Ltd. Glacial acetic acid: Tianjin Yongda Chemical Reagent Co., Ltd. N, N-Dimethylformamide (DMF): Guangdong Guanghua Technology Co., Ltd. Anhydrous ethanol: Yantai City, both Chemical Co., Ltd. The separation and purification of organic solvents used in this industry are industrial grade, other reagents are commercially available analysis. 1H NMR and 13C NMR spectra were measured using a DPX-DPX-400 superconducting nuclear magnetic resonance instrument, TMS as internal standard. High-resolution mass spectrometry was recored using a Waters-Micromass Q-TofMicro high resolution Determination of tetragonal-flight time tandem mass spectrometer.
4-Phenylphthalazin-1(2H)-one (2), 1-chloro-4-phenyl-phthalazine (6) and 1-phenyl-4-(piperazin-1-yl)-phthala-zine (7) were synthesized by reference to the literature [19]. 2-Chloro-N-(phenyl/substituted phenyl)acetamide derivatives were synthesized by by reference to the literature [20].
4.2 Synthesis of 4-phenylphthalazin-1(2H)-thione (3)
4-Phenylphthalazine-1-one (2 mL) was dissolved in a three-necked flask (150 mL) containing acetonitrile (40 mL), and then P2S5 (4 g) was added to the mixture. After reacted for 4 h, the reaction mixture was cooled to room temperature, then the mixture was stirred in cold water (40 mL), then filtering to get the filter cake, putting the filter cake into the sodium hydroxide aqueous solution (100 mL, 1 mol•L-1), and then stirring for 20 min, discarding the residues and getting the filtrate according to filtering, using 10% hydrochloric acid solution to adjust the filtrate to pH=7 and get the precipitation (1.8 g, 84.0% yield).
4.3 General procedure for preparation of 1-phenyl-4-thiophthalazine 5a~5j
0.212 g of compound 3 (839.25 μmol) was added to a round bottom flask (25 mL), then ultra-pure water (5 mL) and 0.033 g of sodium hydroxide (839.25 μmol) were added into the bottom. The mixture was stirred at room temperature to get the clear solution, and then 2-chloro-N-(phenyl/substituted phenyl)acetamide (0.190 g) and anhydrous ethanol (3 mL) were added, respectively. Then the mixture was stirring at room temperature for 8 h. At the end of this period, a solid separated out of the reaction mixture and was collected by filtration. The insoluble solid was washed with anhydrous ethanol (1 mL) and the crude product was recrystallized from ultra-pure water to obtain 5a~5j.
N-(4-Chlorophenyl)-2-((4-phenylphthalazin-1-yl)thio)-acetamide (5a): Yield 50.1%. m.p. 206.6~207.6 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.61 (s, 1H), 8.28 (d, J=8.1 Hz, 1H), 8.07 (dt, J=15.2, 7.1 Hz, 2H), 7.96 (d, J=8.0 Hz, 1H), 7.7~7.62 (m, 4H), 7.63~7.57 (m, 3H), 7.37 (d, J=8.8 Hz, 2H), 4.47 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 166.11, 157.82, 157.22, 137.99, 135.66, 133.56, 133.10, 129.79, 129.23, 128.69, 128.51, 126.87, 126.57, 124.79, 123.65, 123.35, 120.58, 34.45; HRMS (ESI) calcd for C22H16ClN3OSK [M+K]+ 444.0340, found 444.0341.
N-(4-Fluorophenyl)-2-((4-phenylphthalazin-1-yl)thio)-acetamide (5b): Yield 40.4%. m.p. 192.6~193.4 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.60 (s, 1H), 8.28 (d, J=7.9 Hz, 1H), 8.12~8.00 (m, 2H), 7.96 (d, J=7.8 Hz, 1H), 7.78~7.63 (m, 4H), 7.63~7.58 (m, 3H), 7.37 (d, J=8.9 Hz, 2H), 4.47 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 166.11, 157.82, 157.23, 137.99, 135.66, 133.57, 133.11, 129.79, 129.23, 128.69, 128.51, 126.87, 126.57, 124.79, 123.65, 123.35, 120.58, 34.44; HRMS (ESI) calcd for C22H16FN3OSNa [M+Na]+ 412.0896, found 412.0899.
N-(2, 6-Dichlorophenyl)-2-((4-phenylphthalazin-1-yl)-thio)acetamide (5c): Yield 47.5%. m.p. 173.6~176.3 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 8.28 (d, J=7.9 Hz, 1H), 8.08~7.97 (m, 3H), 7.94 (d, J=7.9 Hz, 1H), 7.71 (d, J=4.0 Hz, 2H), 7.59 (dd, J=19.6, 5.7 Hz, 4H), 7.47 (d, J=8.1 Hz, 1H), 7.26 (t, J=7.3 Hz, 1H), 4.68 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 160.49, 159.26, 156.85, 150.77, 135.85, 133.26, 132.85, 131.73, 129.81, 129.14, 128.49, 126.43, 125.75, 124.90, 123.56, 123.46, 121.18, 36.89; HRMS (ESI) calcd for C22H15Cl2N3OSNa [M+ Na]+ 462.0211, found 462.0202.
N-(3, 4-Dichlorophenyl)-2-((4-phenylphthalazin-1-yl)-thio)acetamide (5d): Yield 60.3%. m.p. 182.8~183.4 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.78 (s, 1H), 8.27 (d, J=8.0 Hz, 1H), 8.15~7.99 (m, 3H), 7.96 (d, J=8.0 Hz, 1H), 7.74~7.66 (m, 2H), 7.59 (t, J=6.6 Hz, 4H), 7.52 (dd, J=8.8, 2.2 Hz, 1H), 4.48 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 166.55, 157.71, 157.25, 139.10, 135.64, 133.58, 133.12, 131.03, 130.75, 129.79, 129.24, 128.51, 126.58, 124.78, 123.65, 123.33, 120.20, 119.10, 34.42; HRMS (ESI) calcd for C22H15Cl2N3OSNa [M+ Na]+ 462.0211, found 462.0202.
N-(3-Chloro-4-fluorophenyl)-2-((4-phenylphthalazin-1-yl)thio)acetamide (5e): Yield 70.7%. m.p. 238.6~239.5 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.69 (s, 1H), 8.28 (d, J=8.0 Hz, 1H), 8.07 (dt, J=14.9, 7.1 Hz, 2H), 8.00~7.88 (m, 2H), 7.69 (d, J=3.8 Hz, 2H), 7.60 (d, J=3.6 Hz, 3H), 7.55~7.46 (m, 1H), 7.39 (t, J=9.1 Hz, 1H), 4.47 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 166.29, 157.74, 157.24, 136.29, 136.26, 135.65, 133.56, 133.11, 129.79, 129.23, 128.51, 126.57, 124.79, 123.65, 123.33, 120.41, 117.12, 116.91, 34.35; HRMS (ESI) calcd for C22H16ClFN3OS [M+H]+ 424.0687, found 424.0688.
N-(3-Methoxyphenyl)-2-((4-phenylphthalazin-1-yl)thio)-acetamide (5f): Yield 20.5%. m.p. 185.2~186.0 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.33 (s, 1H), 8.28 (d, J=8.0 Hz, 1H), 8.06 (dt, J=15.1, 7.2 Hz, 2H), 7.95 (d, J=8.0 Hz, 1H), 7.69 (dd, J=6.4, 2.8 Hz, 2H), 7.65~7.57 (m, 3H), 7.53 (d, J=8.9 Hz, 2H), 6.89 (d, J=9.0 Hz, 2H), 4.45 (s, 2H), 3.72 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ:165.37, 157.95, 157.17, 155.26, 135.69, 133.50, 133.06, 132.20, 129.79, 129.21, 128.51, 126.54, 124.82, 123.63, 123.36, 120.59, 113.88, 55.12, 34.39; HRMS (ESI) calcd for C23H20N3O2S [M+H]+ 402.1276, found 402.1278.
2-((4-Phenylphthalazin-1-yl)thio)-N-(o-tolyl)acetamide(5g): Yield 57.3%. m.p. 178.6~179.6 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 9.81 (s, 1H), 8.28 (d, J=7.8 Hz, 1H), 8.11~8.00 (m, 2H), 7.96 (d, J=8.2 Hz, 1H), 7.70 (dd, J=6.0, 2.7 Hz, 2H), 7.66~7.56 (m, 3H), 7.49 (d, J=7.8 Hz, 1H), 7.24~7.12 (m, 2H), 7.07 (t, J=7.3 Hz, 1H), 4.48 (s, 2H), 2.24 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 166.20, 158.06, 157.27, 136.22, 135.71, 133.57, 133.08, 131.24, 130.27, 129.79, 129.24, 128.54, 126.56, 125.93, 125.08, 124.86, 124.40, 123.65, 123.41, 34.01, 17.77. HRMS (ESI) calcd for C23H20N3OS [M+H]+ 386.1327, found 386.1328.
N-(2-Fluoro-4-methylphenyl)-2-((4-phenylphthalazin-1-yl)thio)acetamide (5h): Yield 30.6%. m.p. 230.6~231.5 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.15 (s, 1H), 8.27 (d, J=7.9 Hz, 1H), 8.15~8.01 (m, 2H), 7.96 (d, J=7.9 Hz, 1H), 7.77 (t, J=8.3 Hz, 1H), 7.70 (dd, J=6.4, 3.0 Hz, 2H), 7.64~7.57 (m, 3H), 7.09 (d, J=11.8 Hz, 1H), 6.96 (d, J=8.2 Hz, 1H), 4.51 (s, 2H), 2.27 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 166.37, 157.86, 157.23, 135.68, 133.57, 133.10, 129.80, 129.24, 128.53, 126.57, 124.83, 124.71, 123.65, 123.38, 115.88, 115.69, 33.96, 20.32; HRMS (ESI) calcd for C23H19FN3OS [M+H]+ 404.1233, found 404.1234.
N-(Naphthalen-2-yl)-2-((4-phenylphthalazin-1-yl)thio)-acetamide (5i): Yield 40.9%. m.p. 174.8~175.3 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.01 (s, 1H), 8.28 (d, J=8.0 Hz, 1H), 8.04 (ddd, J=31.7, 19.5, 7.5 Hz, 3H), 7.70 (dd, J=6.5, 2.9 Hz, 2H), 7.66~7.58 (m, 3H), 7.33 (dd, J=6.8, 2.4 Hz, 1H), 7.26~7.11 (m, 2H), 4.48 (s, 2H), 2.20 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 165.98, 157.76, 157.19, 138.40, 135.77, 133.58, 133.52, 133.04, 131.69, 129.78, 129.23, 128.93, 128.56, 127.90, 126.82, 126.51, 124.88, 123.65, 123.46, 33.34, 18.28; HRMS (ESI) calcd for C23H19ClN3OS [M+H]+ 420.0937, found: 420.0936.
N-(2-Chloro-6-methylphenyl)-2-((4-phenyl-phthalazin-1-yl)thio)acetamide (5j): Yield 60.8%. m.p. 253.1~253.7 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.46 (s, 1H), 8.28 (dd, J=18.8, 8.0 Hz, 2H), 8.15~8.02 (m, 2H), 7.96 (dd, J=21.2, 7.8 Hz, 2H), 7.74 (ddd, J=12.9, 11.4, 7.8 Hz, 4H), 7.67~7.60 (m, 3H), 7.56~7.45 (m, 3H), 4.61 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 166.97, 158.13, 157.27, 135.76, 133.66, 133.58, 133.55, 133.10, 129.79, 129.27, 128.57, 128.00, 127.85, 126.58, 126.04, 125.81, 125.54, 125.44, 124.90, 123.68, 123.44, 123.05, 121.58, 34.17; HR-MS (ESI) calcd for C26H20N3OS [M+H]+ 422.1327, found 422.1325.
4.4 Synthesis of 1-(4-(4-chlorobenzyl)piperazin-1-yl)-4-phenylphthalazine (8a)
0.208 g (688.79 μmol) of 1-piperazinyl-4-phenylphth-alazine was added to a single-necked round bottom flask (25 mL), and then N, N-dimethyl formamide (4 mL), 0.055 g (1.38 mmol) of sodium hydroxide and 97 μL (757.67 μmol) of 4-chlorobenzyl chloride were added, respectively. Then the mixture was reacted for 5 h at 140 ℃, and thin layer chromatography (TLC) [V(ethyl acetate):V(petro-leum ether)=3:1] showed the reaction was finished. The mixture was cooled at temperature. The resulting residue was purified by silica gel column chromatography to get a white solid (0.117 g), yield 40.8%. m.p. 218.8~219.8 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.13 (d, J=8.1 Hz, 1H), 8.01 (d, J=8.1 Hz, 1H), 7.82 (t, J=7.6 Hz, 1H), 7.75 (t, J=7.4 Hz, 3H), 7.53 (q, J=6.6 Hz, 3H), 7.38 (d, J=7.8 Hz, 1H), 7.26~7.18 (m, 3H), 3.79 (s, 2H), 3.64 (s, 4H), 2.85 (s, 4H); 13C NMR (101 MHz, CDCl3) δ: 159.37, 156.19, 136.62, 135.67, 134.33, 131.28, 130.92, 130.84, 129.95, 129.45, 128.80, 128.37, 128.22, 127.33, 126.84, 126.69, 124.63, 121.78, 59.27, 53.08, 51.10; HRMS (ESI) calcd for C23H19ClN3OS [M+H]+ 420.0937, found 420.0936.
4.5 Synthesis of 2-(4-(4-phenylnaphthalen-1-yl)-pipezin-1-yl)-N-(2-(trifluoromethyl)phenyl)acetamide(8b)
The preparation method and the feeding ratio are the same as 8a, yield 30.4%. m.p. 252.8~253.7 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.13 (d, J=8.1, 1H), 8.01 (d, J=8.2, 1H), 7.90 (d, J=7.3, 1H), 7.87~7.69 (m, 4H), 7.65 (d, J=7.8, 1H), 7.60~7.44 (m, 4H), 7.36 (t, J=7.5, 1H), 3.82 (s, 2H), 3.63 (s, 4H), 2.82 (s, 4H); 13C NMR (101 MHz, CDCl3) δ: 136.64, 131.88, 131.31, 130.97, 129.97, 128.84, 128.40, 127.37, 126.88, 124.64, 121.85, 58.25, 53.21, 51.17; HRMS (ESI) calcd for C26H24F3N4 [M+H]+ 449.1953, found 449.1952.
4.6 Synthesis of N-(3-chloro-4-fluorophenyl)-2-(4-(4-phenylnaphthalen-1-yl)piperazin-1-yl)acetamide(8c)
0.203 g (688.79 μmol) of 1-piperazinyl-4-phenylphth-alazine was added to a single-necked round bottom flask (25 mL), and then N, N-dimethyl formamide (4 mL), 0.055 g (1.38 mmol) sodium hydroxide and 0.181 g (757.67 μmol) 2-chloro-N-(3-chloro-4-fluorophenyl)acetamide were added. Then the mixture was reacted for 8 h at 140 ℃, and TLC [V(ethyl acetate):V(acetone)=3:4] showed the reaction was finished.The mixture was cooled at temperature.The resulting residue was purified by silica gel column chromatography to get a white solid. yield 33.8%. m.p. 218.8~219.8 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.05 (s, 1H), 8.18 (d, J=8.1 Hz, 1H), 8.05~7.89 (m, 4H), 7.64 (dddd, J=20.5, 17.5, 9.9, 3.5 Hz, 6H), 7.39 (t, J=9.1 Hz, 1H), 3.56 (s, 4H), 3.31 (s, 2H), 2.88 (d, J=10.5 Hz, 4H); 13C NMR (101 MHz, CDCl3) δ: 168.25, 159.37, 156.91, 155.93, 153.48, 136.33, 134.24, 131.66, 131.38, 129.92, 129.06, 128.50, 127.43, 127.09, 124.34, 121.86, 121.64, 121.27, 121.09, 119.13, 119.07, 116.77, 116.55, 61.98, 53.48, 51.26; HRMS (ESI) calcd for C26H24ClFN5O [M+H]+ 476.1653, found 476.1654.
4.7 Effect of compounds on cell viability
Exponentially growing cells were seeded at 4×103 cells per well into 96-well plates. After 24 h incubation at 37 ℃, the culture medium was removed and replaced with fresh medium containing the candidate compounds in different concentrations. The cells were incubated for another 72 h. Then, 20 μL of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) solution (5 mg/mL) was added to all wells and incubated for 4 h at 37 ℃. The medium containing MTT was discarded, 150 μL of dimethyl sulfoxide (DMSO) was added to each well and the plates agitated until the dark blue crystals (formazan) had completely dissolved. The absorbance was measured using a microplate reader at a wavelength of 490 nm. Each concentration was analyzed in triplicate and the experiment was repeated three times. The average 50% inhibitory concentration (IC50) was determined from the concentration-response curves according to the inhibition ratio for each concentration.
Supporting Information The 1H NMR and 13C NMR spectra of 5a~5j and 8a~8c. The Supporting Information is available free of charge via the Internet at http://sioc-journal.cn.
-
-
[1]
For statistical information about cancer, see: World Health Organization. http://www.who.int/mediacentre/factsheets/fs297/zh/.
-
[2]
Curtin, N. Biochem. Soc. Trans. 2014, 42, 82. doi: 10.1042/BST20130187
-
[3]
Bryant, H. E.; Helleday, T. Nucleic Acids Res. 2006, 34, 1685. doi: 10.1093/nar/gkl108
-
[4]
Bryant, H. E.; Schultz, N.; Thomas, H. D.; Parker, K. M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N. J.; Helleday, T. Nature 2005, 434, 913. doi: 10.1038/nature03443
-
[5]
Dillon, K. J.; Smith, G. C.; Martin, N. M. J. Biomol. Screening 2003, 8, 347. doi: 10.1177/1087057103008003013
-
[6]
叶泉英, 国际肿瘤学杂志, 2007, 34, 579. doi: 10.3760/cma.j.issn.1673-422X.2007.08.007Ye, Q. Y. J. Int. Oncol. 2007, 34, 579(in Chinese). doi: 10.3760/cma.j.issn.1673-422X.2007.08.007
-
[7]
Piatnitski, E. L.; Duncton, M. A. J.; Kiselyov, A. S.; Katoch-Rouse, R.; Sherman, D.; Milligan, D. L.; Balagtas, C.; Wong, W. C.; Kawakami, J. Bioorg. Med. Chem. Lett. 2005, 15, 4696. doi: 10.1016/j.bmcl.2005.07.064
-
[8]
Kiselyov, A. S.; Semenov, V. V.; Milligan, D. Chem. Biol. Drug Des. 2006, 68, 308. doi: 10.1111/jpp.2006.68.issue-6
-
[9]
Kiselyov, A. S.; Semenov, V. V.; Milligan, D. Chem. Biol. Drug Des. 2006, 68, 308. doi: 10.1111/jpp.2006.68.issue-6
-
[10]
Zhang, S. L.; Zhao, Y. F.; Liu, Y. J.; Chen, D.; Lan, W. H.; Zhao, Q. L.; Dong, C. C.; Xia, L.; Gong, P. Eur. J. Med. Chem. 2010, 45, 3504. doi: 10.1016/j.ejmech.2010.05.016
-
[11]
Liu, Y. J.; Zhang, S. L.; Li, Y. Wang, J. Q.; Song, Y.; Gong, P. Arch. Pharm. Chem. Life Sci. 2012, 345, 287. doi: 10.1002/ardp.v345.4
-
[12]
王超杰, 曹钦坡, 杨慧, 宋攀攀, 薛登启, 崔飞, 顾一飞, 张孝松, 田亚楠, 张秋荣, 刘宏民, 有机化学, 2016, 36, 1626.Wang, C. J.; Cao, Q. P.; Yang, H.; Song, P. P.; Xue, D. Q.; Cui, F.; Gu, Y. F.; Zhang, X. S.; Tian, Y. N.; Zhang, Q. R.; Liu, H. M. Chin. J. Org. Chem. 2016, 36, 1626(in Chinese).
-
[13]
Hemdan, M. M.; Taha, S. M.; Gabr, A. M.; Elkady, M. Y. J. Chem. Res. 2010, 34, 102. doi: 10.3184/030823410X12658886079090
-
[14]
Grasso, S.; Sarro, G. D.; Sarro, A. D.; Micale, N.; Zappalà, M.; Puja, G.; Baraldi, M.; Micheli, C. D. J. Med. Chem. 2000, 43, 851.
-
[15]
Mertens, M. D.; Pietsch, M.; Schnakenburg, G.; Gütschow, M. J. Org. Chem. 2013, 45, 8966.
-
[16]
Zhang, S. L.; Zhao, Y. F.; Liu Y.; Chen, D.; Lan, W. H.; Zhao, Q. L.; Dong, C. C.; Xia, L.; Gong, P. Eur. J. Med. Chem. 2010, 45, 3504. doi: 10.1016/j.ejmech.2010.05.016
-
[17]
Zhang, Q. R.; Xue, D. Q.; He, P.; Shao, K. P.; Chen, P. J.; Gu, Y. F.; Ren, J. L.; Shan, L. H.; Liu, H. M. Bioorg. Med. Chem. Lett. 2014, 24, 1236. doi: 10.1016/j.bmcl.2013.12.010
-
[18]
Wu, Y.; Sun, L. P.; Ma, L. X.; Chen, J.; Song, M. X.; Cui, X.; Piao, H. R. Chem. Biol. Drug Des. 2013, 81, 591. doi: 10.1111/cbdd.2013.81.issue-5
-
[19]
Abou-Seri, S. M.; Eldehna, W. M.; Ali, M. M.; Abou El Ella, D. A. Eur. J. Med. Chem. 2016, 107, 165. doi: 10.1016/j.ejmech.2015.10.053
-
[20]
Zhou, Z. C.; Shu, W. Y. J. Cent. South Univ. 2002.
-
[1]
-
Table 1. Antitumor activity of target compounds 5a~5j and 8a~8c [IC50/(μmol•L-1)]a
Compound MGC-803 EC-109 PC-3 SMMC-7721 5a 26.43±0.83 40.36±3.20 36.04±1.83 30.67±2.77 5b 32.67±1.47 > 64 > 64 > 64 5c > 64 > 64 49.17±2.96 > 64 5d > 64 58.85±3.67 32.34±1.38 > 64 5e 21.90±1.42 > 64 > 64 44.76±3.46 5f 20.37±2.48 8.13±0.68 28.67±2.47 > 64 5g > 64 > 64 > 64 > 64 5h > 64 > 64 > 64 > 64 5i 50.45±2.83 42.48±0.38 > 64 > 64 5j > 64 40.39±2.84 > 64 > 64 8a > 64 > 64 36.04±1.83 30.67±2.77 8b 32.67±1.47 20.26±0.39 37.96±1.23 42.28±1.71 8c 12.34±0.67 6.31±0.82 20.13±1.06 16.72±0.38 5-Fu 8.41±0.32 14.37±0.29 7.15±0.81 15.79±0.34 a Antitumor activity was assayed by exposure for 72 h to substances and expressed as concentration required to inhibit tumor cell proliferation by 50% (IC50). Dates are presented as the means±SDs of three independent experiments. -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 18
- 文章访问数: 2322
- HTML全文浏览量: 353


 下载:
下载:

 下载:
下载:

