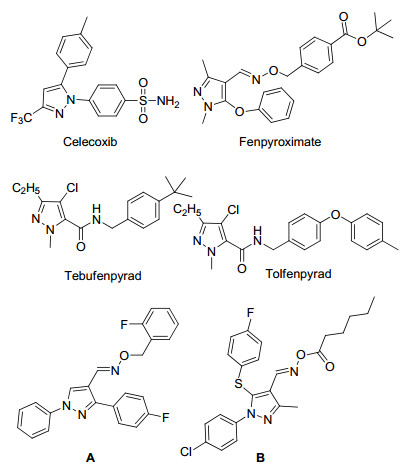
 图1
塞来昔布、唑螨酯、吡螨胺、唑虫酰胺和化合物A~B的化学结构式
Figure1.
Chemical structures of celecoxib, fenpyroximate, tebufenpyrad, tolfenpyrad, and compounds A~B
图1
塞来昔布、唑螨酯、吡螨胺、唑虫酰胺和化合物A~B的化学结构式
Figure1.
Chemical structures of celecoxib, fenpyroximate, tebufenpyrad, tolfenpyrad, and compounds A~B

Citation: Dai Hong, Huang Meiling, Ge Shushan, Sun Siyu, Shen Aibao, Cheng Xiaoyan, Li Chunjian, Shi Jian. Synthesis and Biological Activities of Novel Pyrazole Oxime Esters Containing Substituted Pyrazolyl Group[J]. Chinese Journal of Organic Chemistry, 2017, 37(12): 3289-3295. doi: 10.6023/cjoc201707024

新型含吡唑环结构的吡唑肟酯类化合物的合成与生物活性研究
English
Synthesis and Biological Activities of Novel Pyrazole Oxime Esters Containing Substituted Pyrazolyl Group
-
Key words:
- pyrazol
- / pyrazole oxime
- / synthesis
- / bioactivity
-
随着大量低毒性和高生物活性的杂环先导化合物在文献中被报道, 杂环衍生物渐渐成为了新药研究中的重点.吡唑衍生物具有广谱的生物活性, 是杂环家族中重要的成员之一.经研究发现吡唑衍生物在农业、医药领域极具发展前景.在农药领域, 吡唑衍生物具有杀虫、杀菌及除草等活性[1~5]; 如日本Nihon Nohyaku公司研制开发的杀螨剂唑螨酯(Fenpyroximate)是一种典型的具有良好生物活性的吡唑衍生物, 对于防治多种植食性螨呈现出优异的效果[6, 7].日本三菱化学公司研发的吡唑酰胺类杀虫杀螨剂:吡螨胺(Tebufenpyrad)和唑虫酰胺(Tolfenpyrad), 对螨虫、蚜虫或粘虫等表现出较好的杀灭活性[8, 9].美国Du Pont公司开发的杀虫剂氯虫苯甲酰胺对鳞翅目和半翅目类害虫表现优异的防效[10].在医药领域, 吡唑衍生物表现出良好的抗炎、抗菌与抗肿瘤等活性[11~15], 如塞来昔布(Celecoxib)是一种用于治疗急慢性炎症和关节炎症的新型非甾体抗炎药(NSAIDs).吡唑肟衍生物作为吡唑类化合物中的重要一员, 一直倍受药物研究者的关注[16~20], 如Lv等[21]合成的含有苯环的吡唑肟化合物A显示出对淋巴结细胞和PI3Kγ良好的抑制活性, 其对淋巴结细胞和PI3Kγ细胞系的IC50值分别为1.18和0.28 μmol/L, Ouyang等[22]报道的具有多取代基的吡唑肟化合物B表现出较好的抗烟草花叶病毒活性.鉴于此, 为了进一步寻找与发现具有较好生物活性的吡唑衍生物, 本研究参照唑螨酯的结构, 在保留吡唑肟母核的基础上, 利用活性亚结构拼接原理, 设计并制备了一系列未见文献报道的新型取代吡唑结构的吡唑肟酯衍生物.目标化合物的结构均经1H NMR, 13C NMR和元素分析测试确证.同时对所合成的化合物进行了初步的生物活性测试, 结果表明部分目标化合物具有较好的杀虫效果, 部分目标物还显示出良好的抗肿瘤活性.目标化合物的合成路线如Scheme 1所示.
 图1
塞来昔布、唑螨酯、吡螨胺、唑虫酰胺和化合物A~B的化学结构式
Figure1.
Chemical structures of celecoxib, fenpyroximate, tebufenpyrad, tolfenpyrad, and compounds A~B
图1
塞来昔布、唑螨酯、吡螨胺、唑虫酰胺和化合物A~B的化学结构式
Figure1.
Chemical structures of celecoxib, fenpyroximate, tebufenpyrad, tolfenpyrad, and compounds A~B
1 结果与讨论
1.1 目标化合物的合成
在目标化合物的合成中, 我们以化合物8a的合成作为研究对象, 研究了不同的反应条件对8a收率的影响.研究结果如表 1所示, 其中以二氯甲烷为溶剂, 吡啶为缚酸剂和助溶剂; 室温条件下反应10 h是制备目标化合物8a的较佳方法.化合物8a的收率可达80%.该方法反应条件温和, 而且后续分离简便.采用该方顺利得到了其它化合物, 并通过1H NMR, 13C NMR和元素分析等手段对该系列化合物进行了结构表征.
 表 1
不同反应条件对目标化合物8a合成收率的影响
Table 1.
Effects of reaction conditions on the synthesis of the target compound 8a
表 1
不同反应条件对目标化合物8a合成收率的影响
Table 1.
Effects of reaction conditions on the synthesis of the target compound 8a
Entry Base Solvent Reaction condition Yield/% 1 吡啶 CH2Cl2 r.t. for 10 h 80 2 吡啶 CH3CN r.t. for 10 h 70 3 吡啶 CHCl3 r.t. for 10 h 65 4 Et3N CH2Cl2 r.t. for 10 h 48 5 Et3N CH3CN r.t. for 10 h 60 6 吡啶 CHCl3 Reflux for 10 h 20 7 吡啶 CH3CN Reflux for 10 h 0 1.2 化合物的图谱解析
以目标化合物8c的1H NMR, 13C NMR数据为例进行解析. δ 8.15处的单峰为CH=N上氢的吸收峰; δ 7.32处的双重峰为苯环上两个氢的吸收峰; δ 6.91处的双重峰为苯环上另外两个氢的吸收峰; δ 4.11处的单峰为与吡唑肟相连的吡唑结构上1-位甲基三个氢的吸收峰; δ 3.63处的单峰为吡唑肟中吡唑环1-位甲基上三个氢的吸收峰; δ 2.64处的四重峰为与吡唑环相连的亚甲基上两个氢的吸收峰; δ 2.51处的单峰为与吡唑环相连的甲基上三个氢的吸收峰; δ 1.23处的三重峰为乙基中的甲基上三个氢的吸收峰; δ 156.9处的峰为与羰基碳原子的信号峰; δ 148.9处的峰为CH=N碳原子的信号峰; δ 98.6处的峰为与吡唑环4-位碳原子的信号峰; δ 40.6处的峰为与吡唑环1-位甲基碳原子的信号峰; δ 34.5处的峰为另外一个吡唑环1-位甲基碳原子的信号峰; δ 19.2处的峰为与吡唑环相连的乙基中亚甲基碳原子的信号峰; δ 15.2处的峰对应于乙基中甲基碳原子的信号峰; δ 12.8处的峰对应于另外一个吡唑环3-甲基碳原子的信号峰.
1.3 化合物的杀虫活性
目标化合物8a~8P对蚜虫(Aphis medicaginis)、褐飞虱(Nilaparvata lugens)和粘虫(Oriental armyworm)的杀虫活性测试结果如表 2所示.初步的生物活性测定结果表明, 部分目标物具有良好的杀虫活性.其中当R1=CH3时, 3-氟取代物8b和3-氯取代物8d在测试浓度为500 μg/mL时对蚜虫的杀死率分别为100%和90%, 与对照药吡虫啉的效果相当, 3-氟取代物8b、4-碘取代物8f和4-甲基取代物8j在测试浓度为500 μg/mL时对褐飞虱的杀死率分别为80%, 90%和80%, 与对照药吡虫啉的效果相近, 此外, 3-氯取代物8d和4-三氟甲氧基取代物8h在测试浓度为500 μg/mL时对粘虫表现出一定的防治效果, 其对粘虫的抑制率分别为60%和30%.这为今后进一步进行吡唑肟类化合物的结构优化与生物活性研究提供了重要的实验数据.
 表 2
目标化合物8a~8p的杀虫活性(死亡率/%)a
Table 2.
Insecticidal activities (mortality/%) of target compounds 8a~8p
表 2
目标化合物8a~8p的杀虫活性(死亡率/%)a
Table 2.
Insecticidal activities (mortality/%) of target compounds 8a~8p
Compd. Aphis medicaginis Nilaparvata lugens Oriental armyworm 8a 0 0 0 8b 100 80 0 8c 0 0 0 8d 90 0 60 8e 0 0 0 8f 0 90 0 8g 0 0 0 8h 0 0 30 8i 0 0 0 8j 0 80 0 8k 0 0 0 8l 0 0 0 8m 0 0 0 8n 0 0 0 8o 0 0 0 8p 0 0 0 Imidacloprid 100 100 — Pyridalyl — — 100 a Test concentration of target compounds: 500 μg/mL, — refers to “not tested”. 1.4 化合物的抗肿瘤活性
目标化合物8a~8p对人肝癌(HepG2)和人胃癌(SGC7901)细胞株的体外抗肿瘤活性结果如表 3所示.从表 3可以看出, 目标化合物对SGC7901癌细胞均未表现出明显的抑制效果, 部分化合物对HepG2癌细胞显示出较好的抑制作用, 但无明显的规律.当R1=CH3时, 3-氟取代物8b、4-氯取代物8c、4-溴取代物8e、4-碘取代物8f和4-甲基取代物8j对HepG2细胞的抑制效果相对较好, 其IC50值分别为12.8, 4.1, 2.6, 1.6和11.0 μmol/L, 明显要优于对照药Sorafenib的活性(IC50=16.2 μmol/L).此类化合物的结构衍生与抗肿瘤活性研究正在进行中.
Compd. IC50/(μmol·L-1) HepG2 SGC7901 8a >40 >40 8b 12.8 >40 8c 4.1 >40 8d 16.1 >40 8e 2.6 >40 8f 1.6 >40 8g >40 >40 8h 20.1 >40 8i >40 >40 8j 11.0 >40 8k >40 >40 8l >40 >40 8m 32.8 >40 8n >40 >40 8o >40 >40 8p >40 >40 Sorafenib 16.2 12.1 2 结论
本文采用活性基团拼接原理, 设计并制备了16个新型含吡唑结构的吡唑肟酯类化合物.生物活性测试后发现, 部分化合物具有一定的杀虫活性, 其中化合物8b和8d在测试浓度为500 μg/mL时对蚜虫的杀死率分别为100%和90%, 化合物8b, 8f和8j在测试浓度为500 μg/mL时对褐飞虱的杀死率分别为80%, 90%和80%.此外, 化合物8b, 8c, 8e, 8f和8j对HepG2细胞的抑制效果相对较好, 其IC50值分别为12.8, 4.1, 2.6, 1.6和11.0 μmol/L, 高于对照药Sorafenib的活性.具有进一步研究的价值.
3 实验部分
3.1 仪器与试剂
X-4型数字显示熔点测定仪(北京泰克仪器有限公司), 温度计未经校正; Yanaco-CHN CORDER MT-3自动元素分析仪; Bruker AM-400型核磁共振仪, 以CDCl3为溶剂, TMS为内标; 柱层析硅胶为H型(青岛海洋化工厂, 200~300目).所用试剂均为分析纯.中间体1-甲基-3-乙基-1H-吡唑-5-甲酸乙酯(1)、1-甲基-3-乙基-4-氯-1H-吡唑-5-甲酸乙酯(2)和1-甲基-3-乙基-4-氯-1H-吡唑-5-甲酸(3)按照文献[23, 24]方法制备.中间体1-取代基-3-甲基-5-氯吡唑-4-甲醛(5)、1-取代基-3-甲基-5-取代苯氧基吡唑-4-甲醛(6)和1-取代基-3-甲基-5-取代苯氧基吡唑-4-甲醛肟(7)按照文献[25]方法制备.
3.2 1-甲基-3-乙基-4-氯-1H-吡唑-5-甲酰基氯(4)的合成
在一100 mL反应瓶中, 加入0.03 mol中间体3及0.18 mol二氯亚砜.室温搅拌下, 向其加入几滴DMF, 加热回流6 h, 冷却至室温.减压旋蒸除去过量的二氯亚砜, 得到相应的中间体4, 不经纯化直接用于下一步反应.
3.3 目标化合物8的合成
在一50 mL圆底烧瓶中, 加入5 mmol中间体7, 20 mL无水CH2Cl2及1 mL吡啶.在室温搅拌下, 向其缓慢滴加6 mmol的中间体4的CH2Cl2溶液(10 mL).滴毕, 将反应混合物在室温下反应8~16 h.反应结束后, 向反应液中加入40 mL水, 用二氯甲烷萃取数次, 有机层用无水硫酸钠干燥.抽滤, 减压蒸去溶剂, 所得残余物以石油醚/乙酸乙酯(V:V=20:1)为洗脱剂进行柱层析分离, 得到目标化合物8a~8p.
5-(4-氟苯氧基)-1, 3-二甲基-1H-吡唑-4-甲醛-O-(4-氯-3-乙基-1-甲基-1H-吡唑-5-甲酰基)肟(8a):白色固体, 产率80%. m.p. 128~130 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.13 (s, 1H, CH=N), 6.92~7.08 (m, 4H, ArH), 4.10 (s, 3H, NCH3), 3.64 (s, 3H, NCH3), 2.64 (q, J=8.0 Hz, 2H, CH2), 2.51 (s, 3H, CH3), 1.23 (t, J=8.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 159.7 (d, J=242 Hz), 156.9, 152.4, 150.5, 150.0, 149.0, 148.1, 127.4, 117.2, 117.1, 116.9 (d, J=24 Hz), 112.8, 98.3, 40.6, 34.5, 19.2, 15.2, 12.7. Anal. calcd for C19H19ClFN5O3: C 54.36, H 4.56, N 16.68; found C 54.49, H 4.43, N 16.57.
5-(3-氟苯氧基)-1, 3-二甲基-1H-吡唑-4-甲醛-O-(4-氯-3-乙基-1-甲基-1H-吡唑-5-甲酰基)肟(8b):淡黄色固体, 产率79%. m.p. 100~102 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.18 (s, 1H, CH=N), 6.70~7.35 (m, 4H, ArH), 4.11 (s, 3H, NCH3), 3.63 (s, 3H, NCH3), 2.65 (q, J=6.0 Hz, 2H, CH2), 2.52 (s, 3H, CH3), 1.23 (t, J=8.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 163.6 (d, J=248 Hz), 157.5, 156.9, 150.5, 148.9, 148.2, 131.3, 131.2, 127.4, 112.8, 111.4 (d, J=21 Hz), 111.1, 104.0 (d, J=26 Hz), 98.8, 40.6, 34.6, 19.2, 15.2, 12.8. Anal. calcd for C19H19ClFN5O3: C 54.36, H 4.56, N 16.68; found C 54.25, H 4.67, N 16.80.
5-(4-氯苯氧基)-1, 3-二甲基-1H-吡唑-4-甲醛-O-(4-氯-3-乙基-1-甲基-1H-吡唑-5-甲酰基)肟(8c):淡黄色固体, 产率81%. m.p. 127~129 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.15 (s, 1H, CH=N), 7.32 (d, J=8.0 Hz, 2H, ArH), 6.91 (d, J=8.0 Hz, 2H, ArH), 4.11 (s, 3H, NCH3), 3.63 (s, 3H, NCH3), 2.64 (q, J=8.0 Hz, 2H, CH2), 2.51 (s, 3H, CH3), 1.23 (t, J=8.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 156.9, 155.1, 150.5, 149.5, 148.9, 148.2, 130.2, 129.6, 127.4, 117.0, 112.8, 98.6, 40.6, 34.5, 19.2, 15.2, 12.8. Anal. calcd for C19H19Cl2N5O3: C 52.31, H 4.39, N 16.05; found C 52.19, H 4.28, N 16.17.
5-(3-氯苯氧基)-1, 3-二甲基-1H-吡唑-4-甲醛-O-(4-氯-3-乙基-1-甲基-1H-吡唑-5-甲酰基)肟(8d):白色固体, 产率77%. m.p. 113~115 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.17 (s, 1H, CH=N), 6.83~7.31 (m, 4H, ArH), 4.11 (s, 3H, NCH3), 3.63 (s, 3H, NCH3), 2.64 (q, J=8.0 Hz, 2H, CH2), 2.52 (s, 3H, CH3), 1.23 (t, J=8.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 157.0, 156.9, 150.5, 149.1, 148.9, 148.2, 135.8, 131.0, 127.4, 124.6, 116.4, 113.8, 112.8, 98.8, 40.6, 34.6, 19.2, 15.2, 12.8. Anal. calcd for C19H19Cl2N5O3: C 52.31, H 4.39, N 16.05; found C 52.43, H 4.30, N 16.13.
5-(4-溴苯氧基)-1, 3-二甲基-1H-吡唑-4-甲醛-O-(4-氯-3-乙基-1-甲基-1H-吡唑-5-甲酰基)肟(8e):白色固体, 产率81%. m.p. 132~134 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.15 (s, 1H, CH=N), 7.47 (d, J=8.0 Hz, 2H, ArH), 6.86 (d, J=12.0 Hz, 2H, ArH), 4.11 (s, 3H, NCH3), 3.62 (s, 3H, NCH3), 2.64 (q, J=8.0 Hz, 2H, CH2), 2.51 (s, 3H, CH3), 1.23 (t, J=6.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 156.9, 155.6, 150.5, 149.4, 148.9, 148.2, 133.2, 127.4, 117.4, 117.0, 112.8, 98.6, 40.6, 34.6, 19.2, 15.2, 12.8. Anal. calcd for C19H19BrClN5O3: C 47.47, H 3.98, N 14.57; found C 47.60, H 3.87, N 14.45.
5-(4-碘苯氧基)-1, 3-二甲基-1H-吡唑-4-甲醛-O-(4-氯-3-乙基-1-甲基-1H-吡唑-5-甲酰基)肟(8f):白色固体, 产率78%. m.p. 131~133 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.15 (s, 1H, CH=N), 7.66 (d, J=12.0 Hz, 2H, Ar-H), 6.74 (d, J=8.0 Hz, 2H, ArH), 4.11 (s, 3H, NCH3), 3.62 (s, 3H, NCH3), 2.64 (q, J=8.0 Hz, 2H, CH2), 2.51 (s, 3H, CH3), 1.24 (t, J=8.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 156.9, 156.5, 150.5, 149.3, 148.9, 148.2, 139.2, 127.4, 117.8, 112.8, 98.6, 87.3, 40.6, 34.5, 19.2, 15.2, 12.8. Anal. calcd for C19H19ClIN5O3: C 43.24, H 3.63, N 13.27; found C 43.37, H 3.54, N 13.16.
5-苯氧基-1, 3-二甲基-1H-吡唑-4-甲醛-O-(4-氯-3-乙基-1-甲基-1H-吡唑-5-甲酰基)肟(8g):白色固体, 产率79%. m.p. 130~132 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.14 (s, 1H, CH=N), 6.96~7.39 (m, 5H, ArH), 4.10 (s, 3H, NCH3), 3.62 (s, 3H, NCH3), 2.63 (q, J=8.0 Hz, 2H, CH2), 2.53 (s, 3H, CH3), 1.23 (t, J=8.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 157.0, 156.5, 150.5, 150.1, 149.3, 148.0, 130.3, 127.4, 124.4, 115.8, 112.8, 98.5, 40.6, 34.5, 19.2, 15.4, 12.7. Anal. calcd for C19H20ClN5O3: C 56.79, H 5.02, N 17.43; found C 56.67, H 5.14, N 17.54.
5-(4-三氟甲氧基苯氧基)-1, 3-二甲基-1H-吡唑-4-甲醛-O-(4-氯-3-乙基-1-甲基-1H-吡唑-5-甲酰基)肟(8h):白色固体, 产率81%. m.p. 131~132 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.17 (s, 1H, CH=N), 7.22 (d, J=8.0 Hz, 2H, ArH), 6.98 (d, J=8.0 Hz, 2H, ArH), 4.10 (s, 3H, NCH3), 3.64 (s, 3H, NCH3), 2.65 (q, J=8.0 Hz, 2H, CH2), 2.51 (s, 3H, CH3), 1.23 (t, J=8.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 156.9, 154.7, 150.5, 149.3, 148.8, 148.3, 145.3, 127.4, 123.1, 116.8, 112.8, 98.6, 40.6, 34.6, 19.2, 15.0, 12.7. Anal. calcd for C20H19ClF3N5O4: C 49.44, H 3.94, N 14.41; found C 49.53, H 4.06, N 14.30.
5-(2-三氟甲氧基苯氧基)-1, 3-二甲基-1H-吡唑-4-甲醛-O-(4-氯-3-乙基-1-甲基-1H-吡唑-5-甲酰基)肟(8i):白色固体, 产率71%. m.p. 131~132 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.11 (s, 1H, CH=N), 6.80~7.41 (m, 4H, ArH), 4.10 (s, 3H, NCH3), 3.65 (s, 3H, NCH3), 2.63 (q, J=8.0 Hz, 2H, CH2), 2.53 (s, 3H, CH3), 1.23 (t, J=8.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 156.9, 150.5, 149.1, 148.8, 148.6, 148.2, 137.8, 128.5, 125.0 (q, J=228 Hz), 116.3, 112.9, 98.5, 40.5, 34.5, 19.2, 15.4, 12.7. Anal. calcd for C20H19ClF3N5O4: C 49.44, H 3.94, N 14.41; found C 49.31, H 3.85, N 14.52.
5-(4-甲基苯氧基)-1, 3-二甲基-1H-吡唑-4-甲醛-O-(4-氯-3-乙基-1-甲基-1H-吡唑-5-甲酰基)肟(8j):白色固体, 产率80%. m.p. 100~102 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.11 (s, 1H, CH=N), 7.15 (d, J=8.0 Hz, 2H, ArH), 6.85 (d, J=8.0 Hz, 2H, ArH), 4.10 (s, 3H, NCH3), 3.62 (s, 3H, NCH3), 2.64 (q, J=6.0 Hz, 2H, CH2), 2.52 (s, 3H, CH3), 2.33 (s, 3H, CH3), 1.23 (t, J=8.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 157.0, 154.5, 150.6, 150.5, 149.4, 147.9, 134.1, 130.7, 127.5, 115.7, 112.8, 98.2, 40.6, 34.5, 20.6, 19.2, 15.4, 12.8. Anal. calcd for C20H22ClN5O3: C 57.76, H 5.33, N 16.84; found C 57.63, H 5.42, N 16.96.
5-(3-甲基苯氧基)-1, 3-二甲基-1H-吡唑-4-甲醛-O-(4-氯-3-乙基-1-甲基-1H-吡唑-5-甲酰基)肟(8k):白色固体, 产率75%. m.p. 107~109 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.14 (s, 1H, CH=N), 6.74~7.23 (m, 4H, ArH), 4.11 (s, 3H, NCH3), 3.61 (s, 3H, NCH3), 2.64 (q, J=8.0 Hz, 2H, CH2), 2.53 (s, 3H, CH3), 2.35 (s, 3H, CH3), 1.23 (t, J=8.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 157.0, 156.6, 150.5, 150.3, 149.4, 148.0, 140.8, 130.0, 127.5, 125.2, 116.4, 112.8, 98.5, 40.6, 34.5, 21.4, 19.2, 15.4, 12.8. Anal. calcd for C20H22ClN5O3: C 57.76, H 5.33, N 16.84; found C 57.87, H 5.21, N 16.73.
5-(2, 4-二氟苯氧基)-1, 3-二甲基-1H-吡唑-4-甲醛-O-(4-氯-3-乙基-1-甲基-1H-吡唑-5-甲酰基)肟(8l):黄色固体, 产率76%. m.p. 127~129 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.12 (s, 1H, CH=N), 6.81~7.02 (m, 3H, ArH), 4.11 (s, 3H, NCH3), 3.70 (s, 3H, NCH3), 2.64 (q, J=6.0 Hz, 2H, CH2), 2.48 (s, 3H, CH3), 1.24 (t, J=8.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 156.8, 150.5, 149.7, 148.6, 148.2, 127.4, 118.8, 118.7, 112.8, 111.5 (d, J=23 Hz), 106.1 (d, J=22 Hz), 105.8 (d, J=21 Hz), 97.7, 40.5, 34.5, 19.2, 14.9, 12.8. Anal. calcd for C19H18ClF2N5O3: C 52.12, H 4.14, N 16.00; found C 52.01, H 4.23, N 16.12.
5-(2, 4-二氯苯氧基)-1, 3-二甲基-1H-吡唑-4-甲醛-O-(4-氯-3-乙基-1-甲基-1H-吡唑-5-甲酰基)肟(8m):白色固体, 产率80%. m.p. 129~130 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.12 (s, 1H, CH=N), 6.74~7.51 (m, 3H, ArH), 4.11 (s, 3H, NCH3), 3.66 (s, 3H, NCH3), 2.64 (q, J=6.0 Hz, 2H, CH2), 2.51 (s, 3H, CH3), 1.24 (t, J=8.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 156.8, 150.7, 154.5, 149.1, 148.6, 148.2, 131.0, 132.2, 128.3, 127.3, 124.2, 117.3, 112.9, 98.4, 40.6, 34.5, 19.2, 15.1, 12.8. Anal. calcd for C19H18Cl3N5O3: C 48.48, H 3.85, N 14.88; found C 48.61, H 3.73, N 14.77.
5-(4-氟苯氧基)-1-(4-甲基苯基)-3-甲基-1H-吡唑-4-甲醛-O-(4-氯-3-乙基-1-甲基-1H-吡唑-5-甲酰基)肟(8n):黄色固体, 产率70%. m.p. 103~105 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.19 (s, 1H, CH=N), 6.89~7.48 (m, 8H, ArH), 4.11 (s, 3H, NCH3), 2.64 (q, J=6.0 Hz, 2H, CH2), 2.60 (s, 3H, CH3), 2.35 (s, 3H, CH3), 1.24 (t, J=8.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 159.1 (d, J=242 Hz), 156.9, 152.5, 152.4, 150.5, 149.4, 149.1, 148.9, 137.9, 134.7, 129.8, 127.4, 122.4, 117.4, 117.3, 116.7 (d, J=24 Hz), 112.8, 99.8, 40.6, 21.1, 19.3, 15.4, 12.8. Anal. calcd for C25H23ClFN5O3: C 60.55, H 4.67, N 14.12; found C 60.65, H 4.55, N 14.01.
5-(4-氯苯氧基)-1-(4-甲基苯基)-3-甲基-1H-吡唑-4-甲醛-O-(4-氯-3-乙基-1-甲基-1H-吡唑-5-甲酰基)肟(8o):黄色固体, 产率71%. m.p. 114~115 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.21 (s, 1H, CH=N), 6.87~7.47 (m, 8H, ArH), 4.12 (s, 3H, NCH3), 2.65 (q, J=8.0 Hz, 2H, CH2), 2.60 (s, 3H, CH3), 2.34 (s, 3H, CH3), 1.24 (t, J=8.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 156.9, 155.1, 150.5, 149.2, 148.8, 137.9, 134.6, 130.1, 129.9, 129.4, 127.4, 122.4, 117.1, 112.9, 100.1, 40.6, 21.1, 19.2, 15.3, 12.8. Anal. calcd for C25H23Cl2N5O3: C 58.60, H 4.52, N 13.67; found C 58.47, H 4.63, N 13.79.
5-(4-甲基苯氧基)-1-(4-甲基苯基)-3-甲基-1H-吡唑-4-甲醛-O-(4-氯-3-乙基-1-甲基-1H-吡唑-5-甲酰基)肟(8p):黄色固体, 产率77%. m.p. 133~134 ℃; 1H NMR (400 MHz, CDCl3) δ: 8.14 (s, 1H, CH=N), 7.50 (d, J=8.0 Hz, 2H, ArH), 7.18 (d, J=8.0 Hz, 2H, ArH), 7.09 (d, J=8.0 Hz, 2H, ArH), 6.85 (d, J=8.0 Hz, 2H, ArH), 4.11 (s, 3H, NCH3), 2.64 (q, J=8.0 Hz, 2H, CH2), 2.60 (s, 3H, CH3), 2.34 (s, 3H, CH3), 2.29 (s, 3H, CH3), 1.24 (t, J=8.0 Hz, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ: 156.9, 154.6, 150.5, 149.9, 149.4, 149.0, 137.6, 134.8, 134.0, 130.6, 129.7, 127.4, 122.3, 115.9, 112.8, 99.6, 40.5, 21.1, 20.6, 19.2, 15.6, 12.8. Anal. calcd for C26H26ClN5O3: C 63.48, H 5.33, N 14.24; found C 63.36, H 5.43, N 14.35.
3.4 生物活性测试
用分析天平称取一定质量的原药, 用含吐温-80乳化剂的DMF溶解配制成1.0%母液, 然后用蒸馏水稀释备用.每个处理3次重复, 设空白对照.杀虫活性测试所选昆虫分别为粘虫(Oriental armyworm)、蚜虫(Aphis medicaginis)和褐飞虱(Nilaparvata lugens).对照药剂分别为啶虫丙醚(Pyridalyl)和吡虫啉(Imidacloprid).
蚜虫和褐飞虱采用喷雾法.首先, 分别将接有蚜虫的蚕豆叶片和接有褐飞虱的水稻苗于Potter喷雾塔下喷雾处理, 处理后蚜虫置于20~22 ℃观察室内培养, 褐飞虱置于24~27 ℃观察室内培养, 调查药后2 d的死活虫数, 并进行统计分析.
粘虫采用浸叶碟法.首先, 将适量玉米叶在配好的药液中充分浸润后自然阴干, 放入垫有滤纸的培养皿中, 接粘虫3龄中期幼虫10头/皿, 置于24~27 ℃观察室内培养, 调查药后2 d的死活虫数, 并进行统计分析.
抗肿瘤活性测试所用细胞株分别为人肝癌(HepG2)和人胃癌(SGC7901)细胞株.阳性对照药为索拉菲尼(Sorafenib).采用MTT法测定了目标化合物的体外抗肿瘤活性, 具体方法参照文献[26].
辅助材料(Supporting Information) 化合物8a~8p的1H NMR和13C NMR图谱.这些材料可以免费从本刊网站(http://sioc-journal.cn/)上下载.
-
-
[1]
王宏青, 刘惠, 刘钊杰, 有机化学, 2004, 24, 1563. doi: 10.3321/j.issn:0253-2786.2004.12.008Wang, H. Q.; Liu, H.; Liu, Z. J. Chin. J. Org. Chem. 2004, 24, 1563(in Chinese). doi: 10.3321/j.issn:0253-2786.2004.12.008
-
[2]
王宏青, 刘惠, 刘钊杰, 有机化学, 2004, 24, 797. doi: 10.3321/j.issn:0253-2786.2004.07.014Wang, H. Q.; Liu, H.; Liu, Z. J. Chin. J. Org. Chem. 2004, 24, 797(in Chinese). doi: 10.3321/j.issn:0253-2786.2004.07.014
-
[3]
谭成侠, 沈德隆, 翁建全, 欧晓明, 有机化学, 2005, 25, 1268. doi: 10.3321/j.issn:0253-2786.2005.10.018Tan, C. X.; Shen, D. L.; Weng, J. Q. Ou, X. M. Chin. J. Org. Chem. 2005, 25, 1268(in Chinese). doi: 10.3321/j.issn:0253-2786.2005.10.018
-
[4]
Zhou, Z. Z.; Yang, G. F. Bioorg. Med. Chem. 2006, 14, 8666. doi: 10.1016/j.bmc.2006.08.020
-
[5]
Wu, Z. B.; Zhou, X.; Ye, Y. Q.; Wang, P. Y.; Yang, S. Chin. Chem. Lett. 2017, 28, 121. doi: 10.1016/j.cclet.2016.06.010
-
[6]
Hamaguchi, H.; Kajihara, O.; Katoh, M. J. Pestic. Sci. 1995, 20, 173. doi: 10.1584/jpestics.20.173
-
[7]
Swanson, M. B.; Ivancic, W. A.; Saxena, A. M.; Allton, J. D.; O'Brien, G. K.; Suzuki, T.; Nishizawa, H.; Nokata, M. J. Agric. Food Chem. 1995, 43, 513. doi: 10.1021/jf00050a048
-
[8]
顾保权, 朱伟清, 范文政, 钱虹, 刘建梅, 张爱庆, 沈荣仙, 现代农药, 2002, 1, 9. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=nyxd200206002&dbname=CJFD&dbcode=CJFQGu, B. Q.; Zhu, W. Q.; Fan, W. Z.; Qian, H.; Liu, J. M.; Zhang, A. Q.; Shen, R. X. Mod. Agrochem. 2002, 1, 9(in Chinese). http://kns.cnki.net/KCMS/detail/detail.aspx?filename=nyxd200206002&dbname=CJFD&dbcode=CJFQ
-
[9]
范文政, 顾保权, 朱伟, 张一宾, 现代农药, 2005, 4, 9. doi: 10.3969/j.issn.1671-5284.2005.02.002Fan, W. Z.; Gu, B. Q.; Zhu, W.; Zhang, Y. B. Mod. Agrochem. 2005, 4, 9(in Chinese). doi: 10.3969/j.issn.1671-5284.2005.02.002
-
[10]
Lahm, G. P.; Selby T. P.; Freudenberger, J. H.; Stevenson, T. M.; Myers, B. J.; Seburyamo, G.; Smith, B. K.; Flexner, L.; Clark, C. E.; Cordova, D. Bioorg. Med. Chem. Lett. 2005, 15, 4898. doi: 10.1016/j.bmcl.2005.08.034
-
[11]
El-Tamany, E. S.; El-Shahed, F. A.; Mohamed, B. H. J. Serb. Chem. Soc. 1999, 64, 9.
-
[12]
Wang, J. L.; Liu, D. X.; Zhang, Z. J.; Shan, S.; Han, X. B.; Srinivasula, S. M.; Croce, C. M.; Alnemri, E. S.; Huang, Z. W. Proc. Natl. Acad. Sci. U. S. A. 2000, 97, 7124. doi: 10.1073/pnas.97.13.7124
-
[13]
Zakia, M. E. A.; Solimana, H. A.; Hiekalb, Ola. A.; Rashada, A. E. Z. Naturforsch. C 2006, 61, 1. http://www.ncbi.nlm.nih.gov/pubmed/16610208
-
[14]
Liang, X.; Zang, J.; Zhu, M.; Gao, Q.; Wang, B.; Xu, W.; Zhang, Y. ACS Med. Chem. Lett. 2016, 7, 950. doi: 10.1021/acsmedchemlett.6b00247
-
[15]
Li, J.; Huo, H.; Guo, R.; Liu, B.; Li, L.; Dan, W.; Xiao, X.; Zhang, J.; Shi, B. Eur. J. Med. Chem. 2017, 130, 1. doi: 10.1016/j.ejmech.2017.02.033
-
[16]
Ouyang, G. P.; Cai, X. J.; Chen, Z.; Song, B. A.; Bhadury, P. S.; Yang, S.; Jin, L. H.; Xue, W.; Hu, D. Y.; Zeng, S. J. Agric. Food Chem. 2008, 56, 10160. doi: 10.1021/jf802489e
-
[17]
Dai, H.; Xiao, Y. S.; Li, Z.; Xu, X. Y.; Qian, X. H. Chin. Chem. Lett. 2014, 25, 1014. doi: 10.1016/j.cclet.2014.06.011
-
[18]
Fu, C. R.; Peng, J.; Ning, Y.; Liu, M.; Shan, P. C.; Liu, J.; Li, Y. Q.; Hu, F. Z.; Zhu, Y. Q.; Yang, H. Z.; Zou, X. M. Pest Manage. Sci. 2014, 1207. http://www.ncbi.nlm.nih.gov/pubmed/24167146
-
[19]
Wang, S. L.; Shi, Y. J.; He, H. B.; Li, Y.; Li, Y.; Dai, H. Chin. Chem. Lett. 2015, 26, 672. doi: 10.1016/j.cclet.2015.04.017
-
[20]
Dai, H.; Ge, S. S.; Li, G.; Chen, J.; Shi, Y. J.; Ye, L. Y.; Ling, Y. Bioorg. Med. Chem. Lett. 2016, 26, 4504. doi: 10.1016/j.bmcl.2016.07.068
-
[21]
Lv, X. H.; Li, Q. S.; Ren, Z. L.; Chu, M. J.; Sun, J.; Zhang, X.; Xing, M.; Zhu, H. L.; Cao, H. Q. Eur. J. Med. Chem. 2015, 108, 586. http://www.ncbi.nlm.nih.gov/pubmed/26720154
-
[22]
Ouyang, G.; Chen, Z.; Cai, X. J.; Song, B. A.; Bhadury, P. S.; Yang, S.; Jin, L. H.; Xue, W.; Hu, D. Y.; Zeng, S. Bioorg. Med. Chem. 2008, 16, 9699. doi: 10.1016/j.bmc.2008.09.070
-
[23]
Wei, F.; Zhao, B. X.; Huang, B.; Zhang, L.; Sun, C. H.; Dong, W. L.; Shin, D. S.; Miao, J. Y. Bioorg. Med. Chem. Lett. 2006, 16, 6342. doi: 10.1016/j.bmcl.2006.09.008
-
[24]
Song, H. J.; Liu, Y. X.; Xiong, L. X.; Li, Y. Q.; Yang, N.; Wang, Q. M. J. Agric. Food Chem. 2013, 61, 8730. doi: 10.1021/jf402719z
-
[25]
Park, H. J.; Lee, K.; Park, S. J.; Ahn, B.; Lee, J. C.; Cho, H. Y.; Lee, K. I. Bioorg. Med. Chem. Lett. 2005, 15, 3307. doi: 10.1016/j.bmcl.2005.03.082
-
[26]
石玉军, 李阳, 方源, 叶林玉, 陈佳, 戴红, 有机化学, 2016, 36, 1431. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=yjhu201606028&dbname=CJFD&dbcode=CJFQShi, Y. J.; Li, Y.; Fang, Y.; Ye, L. Y.; Chen, J.; Dai, H. Chin. J. Org. Chem. 2016, 36, 1431(in Chinese). http://kns.cnki.net/KCMS/detail/detail.aspx?filename=yjhu201606028&dbname=CJFD&dbcode=CJFQ
-
[1]
-
表 1 不同反应条件对目标化合物8a合成收率的影响
Table 1. Effects of reaction conditions on the synthesis of the target compound 8a
Entry Base Solvent Reaction condition Yield/% 1 吡啶 CH2Cl2 r.t. for 10 h 80 2 吡啶 CH3CN r.t. for 10 h 70 3 吡啶 CHCl3 r.t. for 10 h 65 4 Et3N CH2Cl2 r.t. for 10 h 48 5 Et3N CH3CN r.t. for 10 h 60 6 吡啶 CHCl3 Reflux for 10 h 20 7 吡啶 CH3CN Reflux for 10 h 0 表 2 目标化合物8a~8p的杀虫活性(死亡率/%)a
Table 2. Insecticidal activities (mortality/%) of target compounds 8a~8p
Compd. Aphis medicaginis Nilaparvata lugens Oriental armyworm 8a 0 0 0 8b 100 80 0 8c 0 0 0 8d 90 0 60 8e 0 0 0 8f 0 90 0 8g 0 0 0 8h 0 0 30 8i 0 0 0 8j 0 80 0 8k 0 0 0 8l 0 0 0 8m 0 0 0 8n 0 0 0 8o 0 0 0 8p 0 0 0 Imidacloprid 100 100 — Pyridalyl — — 100 a Test concentration of target compounds: 500 μg/mL, — refers to “not tested”. 表 3 目标化合物8a~8p的体外抗肿瘤活性
Table 3. Cytotoxicity of title compounds 8a~8p
Compd. IC50/(μmol·L-1) HepG2 SGC7901 8a >40 >40 8b 12.8 >40 8c 4.1 >40 8d 16.1 >40 8e 2.6 >40 8f 1.6 >40 8g >40 >40 8h 20.1 >40 8i >40 >40 8j 11.0 >40 8k >40 >40 8l >40 >40 8m 32.8 >40 8n >40 >40 8o >40 >40 8p >40 >40 Sorafenib 16.2 12.1 -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 7
- 文章访问数: 2610
- HTML全文浏览量: 292



 下载:
下载:

 下载:
下载:

