 图1
嘧啶类抗肿瘤药物的结构式
Figure1.
Structures of pyrimidines antitumor
图1
嘧啶类抗肿瘤药物的结构式
Figure1.
Structures of pyrimidines antitumor

Citation: Song Panpan, Li Na, Cui Fei, Xin Jingchao, Zhang Xiaosong, Cao Qinpo, Wang Chaojie, Dai Wenjie, Meng Xiangchuan, Liu Meng, Chang Tonghang, Liu Qingyi, Sun Yuehonga, Ke Yu, Zhang Qiurong, Liu Hongmin. Synthesis and Antitumor Activity Evaluation of 2, 4, 6-Trisubstituted Pyrimidine Derivatives[J]. Chinese Journal of Organic Chemistry, 2017, 37(10): 2725-2735. doi: 10.6023/cjoc201705013

2, 4, 6-三取代嘧啶衍生物的合成及体外抗肿瘤活性研究
-
关键词:
- 2, 4, 6-三取代嘧啶
- / 查尔酮
- / 抗肿瘤
English
Synthesis and Antitumor Activity Evaluation of 2, 4, 6-Trisubstituted Pyrimidine Derivatives
-
Key words:
- 2, 4, 6-trisubstituted pyrimidine
- / chalcone
- / antitumor
-
根据由世界卫生组织下属的官方癌症机构国际癌症研究中心(IARC)编撰最新版的《世界癌症报告》预测, 全球癌症病例将呈现迅猛增长态势, 将由2012年的1400万人, 逐年递增至2025年的1900万人, 到2035年将达到2400万人. 2015年, 美国食品药品管理局(FDA)批准了45种新药, 其中14例(31.11%)用于治疗癌症[1].嘧啶杂环类化合物广泛地存在于自然界生物体内, 参与不同的生命活动, 是一种特别重要的生物内源物质, 对生物体的生命活动起到重要作用.通常具有嘧啶环结构单元的化合物分子都具有广泛的生物和药理活性, 例如具有抗肿瘤作用的5-氟尿嘧啶, 具有抗人类免疫缺陷病毒(HIV)的齐多夫定、具有抗病毒作用的碘苷、具有抗菌作用的美替普林和溴莫普林、具有抗甲状腺的丙硫氧嘧啶以及具有镇静和抗惊厥作用的苯巴比妥等[2, 3].因此, 嘧啶作为药物分子设计和合成的基本砌块已引起人们的广泛关注, 目前仍是新药分子设计、合成与生物活性研究中的一个十分活跃的领域.许多论文报道, 嘧啶衍生物具有良好的抗癌活性[4~7]. 1957年, Duschinsky等[8]首次合成了第一代氟化嘧啶药物5-氟尿嘧啶(1), 这对于肿瘤化疗治疗具有划时代的意义, 是现代癌症化疗的重要里程碑. 1967年, 人类首次合成了可以口服的非肿瘤选择性药物替加氟(2). 1992年肿瘤内激活的靶向性口服药物氟脲苷(3)和卡培他滨(4)等都已经成功上市(图 1)[9, 10].
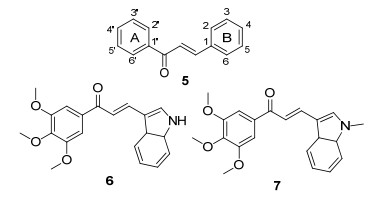
另一方面, 查尔酮(5)是由烯酮连接的两个芳环构成, 其结构的特殊性导致其具有柔性, 从而能与不同的生物受体结合, 也正是基于这个原因以及其本身的生物活性, 无数的科研工作者将之应用于各类药物的研究中, 得到了意想不到的成效, 尤其在抗肿瘤、抗疟疾、抗HIV、抗菌、食用等方面得到了令人惊喜的研究成果与进展[11~14]. Sharma等[15]设计合成了一系列吲哚基查尔酮类衍生物, 并对其体外抗肿瘤活性进行评价, 活性较好的化合物6和化合物7(图 2)对PaCa-2癌细胞系的IC50值分别为0.03和0.09 μmol/L, 且具有良好的选择性, 被认为是最具有潜力的抗癌药物.鉴于此, 我们根据生物活性亚结构拼接原理, 以嘧啶环为基本母核, 设计并合成了一系列具有查尔酮官能团的2, 4, 6-三取代嘧啶衍生物(Scheme 1), 并通过有效的合成途径将取代基R1引入到C-6位, R2引入到C-4位, 进一步在体外评价了其对人类癌细胞系MGC-803人胃癌细胞系、HepG-2人肝癌细胞系、EC-109人食管癌细胞系和MDA-MB-231乳腺癌细胞系的细胞毒活性.
1 结果与讨论
1.1 目标化合物的合成
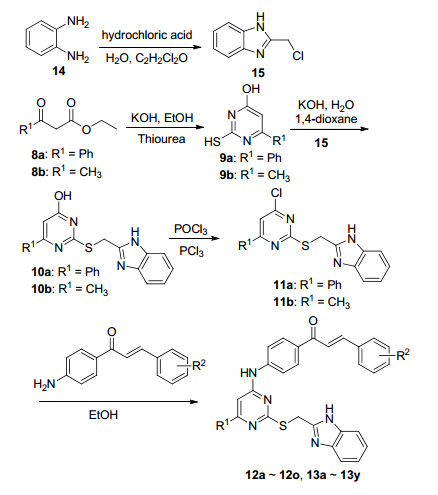
目标化合物以乙酰乙酸乙酯或苯甲酰基乙酸乙酯为起始原料经四步反应获得.合成路线如Scheme 1所示, 我们以苯甲酰乙酸乙酯(8a)或乙酰乙酸乙酯(8b)和硫脲为起始原料, 以无水乙醇为溶剂, 在氢氧化钾存在条件下, 85 ℃回流5 h发生环合反应, 以较高的产率合成了化合物9a和9b, 进而以水和1, 4-二氧六环为混合溶剂, 将9a和9b与2-氯亚甲基-1H-苯并[d]咪唑缩合得到化合物10a, 10b.随后以POCl3和PCl3作为氯代试剂, 化合物10a, 10b发生氯代反应, 得到化合物11a, 11b[16, 17].然后将高活化中间体11a和11b与不同取代的查尔酮反应, 分别得到化合物12a~12o和13a~13y.
1.2 化合物的生物活性分析
采用CCK-8[18]比色法考察40个目标化合物对体外培养的四种癌细胞MGC-803人胃癌细胞、HepG-2人肝癌细胞、EC-109人食管癌细胞和MDA-MB-231乳腺癌细胞生长的抑制作用(表 1).
 表 1
化合物12a~12o和13a~13y的抗肿瘤活性[IC50/(μmol•L-1)]a
Table 1.
Antitumor activity [IC50/(μmol•L-1)] of target compounds 12a~12o and 13a~13y
表 1
化合物12a~12o和13a~13y的抗肿瘤活性[IC50/(μmol•L-1)]a
Table 1.
Antitumor activity [IC50/(μmol•L-1)] of target compounds 12a~12o and 13a~13y
Compd. R1 R2 MGC-803 HepG-2 EC-109 MDA-MB-231 12a Ph H >100 33.90±1.60 36.66±1.02 >100 12b Ph 2-F >100 >100 >100 >100 12c Ph 3-F >100 >100 >100 >100 12d Ph 4-F >100 20.39±1.67 55.37±1.65 >100 12e Ph 3, 4-F2 >100 >100 >100 10.89±0.22 12f Ph 3, 5-F2 21.67±0.87 27.69±0.63 18.70±0.94 4.11±0.34 12g Ph 4-Cl >100 >100 23.00±1.24 19.90±1.13 12h Ph 3, 5-Cl2 24.82±1.32 9.75±1.81 20.67±1.26 6.45±0.65 12i Ph 2-Br 65.83±1.65 76.35±1.85 >100 16.90±0.64 12j Ph 3-Br >100 44.50±1.36 >100 >100 12k Ph 4-Br >100 >100 >100 >100 12l Ph 3, 5-(OCH3)2 >100 >100 >100 >100 12m Ph 4-HO >100 >100 >100 >100 12n Ph 3-NO2 >100 >100 >100 >100 12o Ph 2-Thienyl >100 >100 >100 45.86±1.54 13a CH3 H >100 >100 >100 >100 13b CH3 2-F 40.12±1.36 31.46±1.14 44.50±0.90 19.99±0.65 13c CH3 3-F 5.45±1.85 20.67±1.38 19.24±1.35 8.86±1.27 13d CH3 4-F 7.01±0.09 9.92±0.36 10.87±0.99 9.99±0.86 13e CH3 3, 4-F2 13.42±0.50 13.41±0.52 19.80±1.20 15.00±0.71 13f CH3 3, 5-F2 7.15±1.43 8.37±0.97 14.41±0.94 2.63±0.46 13g CH3 4-Cl >100 >100 >100 33.08±0.58 13h CH3 2, 4-Cl2 9.85±0.96 33.33±0.33 40.29±0.69 8.68±0.08 13i CH3 2, 5-Cl2 >100 82.59±0.62 >100 31.03±0.55 13j CH3 3, 5-Cl2 25.45±1.11 >100 >100 >100 13k CH3 2-Br 19.14±1.11 6.80±1.05 25.90±1.22 2.83±0.88 13l CH3 3-Br 20.64±0.79 4.89±1.21 22.72±0.93 10.67±0.66 13m CH3 4-Br 3.19±0.07 10.05±0.06 11.99±1.19 12.46±0.66 13n CH3 2-F-4-Br 7.61±1.24 28.53±1.74 17.04±1.26 17.50±1.03 13o CH3 4-F-3-Br 2.90±1.36 15.94±1.35 23.17±1.37 6.69±0.76 13p CH3 4-CH3- 14.60±1.13 24.19±0.80 33.11±1.19 17.94±0.87 13q CH3 4-CH(CH3)2 41.71±1.07 35.54±1.23 50.21±1.36 48.73±0.12 13r CH3 4-C(CH3)3 15.95±1.84 36.41±1.88 25.00±0.83 10.91±0.08 13s CH3 3-HO 13.67±0.23 18.66±0.91 20.76±1.05 6.77±0.84 13t CH3 2, 3-(OCH3)2 10.62±0.95 18.33±1.31 58.80±1.52 25.85±1.43 13u CH3 3, 4, 5-(OCH3)3 0.99±0.19 21.19±0.86 20.43±1.04 1.77±0.27 13v CH3 4-OH-3-OCH3 37.88±1.40 >100 >100 >100 13w CH3 3-NO2 1.43±0.15 2.20±0.38 16.71±0.51 3.02±0.21 13x CH3 2-Thienyl 4.05±0.51 5.27±0.88 12.80±0.64 1.05±0.14 13y CH3 2-Furyl 3.49±0.69 13.05±0.85 23.67±1.30 2.14±0.21 5-Fub 7.96± 0.87 10.36±0.28 16.64±1.12 6.58±0.38 a Antitumor activity was assayed by exposure for 72 h to substances and expressed as concentration required to inhibit tumor cell proliferation by 50% (IC50). Dates are presented as the means±SDs of three independent experiments. b Positive control. 从表 1中的数据可以得出, 化合物12a~12o中绝大多数对这四种肿瘤细胞都不具有抑制活性, 遂将R1上的苯基取代基(12a~12o)改造为甲基取代基(13a~13y)期待得到活性更好的化合物.由化合物12a~12o和13a~13y的生物活性结果可以看出, 13a~13y大多数化合物对以上四种癌细胞系均有良好的抑制活性, 尤其是对于MGC-803, MDA-MB-231这两个癌细胞株活性更佳.通过对比, 影响此类化合物生物活性的重要因素除了查尔酮结构中B环上的取代基之外, 嘧啶环上6位取代基对其活性影响特别大, 是决定其生物活性尤其是抗肿瘤活性的关键因素.当R1=CH3时的生物活性明显优于R1=Ph时的生物活性, 说明嘧啶环上6位小体积的给电子取代基团更有利于化合物抗肿瘤活性的表达.当R1=CH3时, (1) 比较化合物13a与其他化合物, 发现查尔酮结构B环上没有取代基时无活性, 存在取代基有利于其活性的提高; (2) 比较化合物13p, 13q, 13r和13m, 13d, 发现当查尔酮B环的对位为吸电子基团的卤素时, 更有利于化合物抗肿瘤活性的表达; (3) 分别比较13d, 13f与之对应的13g, 13j, 发现同为吸电子基团取代, F原子活性要较Cl原子取代时高; (4) 比较13h和13i, 13j, 同为查尔酮B环二氯取代, 氯原子在邻对位时活性优于在邻位、间位或全在间位的化合物; (5) 化合物13d和13m的抗肿瘤活性优于13b, 13c和13k, 13l, 说明同为吸电子卤素单取代时, 取代基处于对位时要优于邻位和间位; (6) 以化合物13x, 13y作为尝试, 将查尔酮的B环变成2-噻吩环或2-呋喃环, 发现这种5元杂环对于抗肿瘤活性是有利的.由体外抗肿瘤活性评价实验结果发现, 其中化合物13f, 13u, 13w, 13x对上述四种癌细胞的抑制作用均优于或接近阳性对照5-氟尿嘧啶.其中化合物13x, 对以上四种细胞的IC50值分别为4.05, 5.27, 12.80和1.05 μmol•L-1, 化合物13u对MGC-803和MDA-MB-231细胞株的IC50值分别为0.99和1.77 μmol•L-1.
2 结论
设计并制备具有查尔酮官能团的2, 4, 6-三取代嘧啶衍生物, 利用核磁共振氢谱、核磁共振碳谱和高分辨质谱等技术证实了目标化合物的结构.运用CCK-8比色法测定了目标化合物对人类四种癌细胞MGC-803人胃癌细胞、HepG2人肝癌细胞、EC-109人食管癌细胞和MDA-MB-231乳腺癌细胞的抗肿瘤活性, 其中化合物13u对MGC-803和MDA-MB-231细胞株抗肿瘤活性要优于5-氟尿嘧啶, 其IC50值分别为0.99和1.77 μmol• L-1.
3 实验部分
3.1 仪器与试剂
1H NMR和13C NMR谱使用瑞典Bruker公司DPX-400型超导核磁共振仪测定, TMS为内标; 高分辨质谱使用美国Waters-Micromass公司Q-TofMicro高分辨四极杆-飞行时间串联质谱仪测定.薄层硅胶:中国青岛海洋化工集团公司, 柱层析硅胶:上海五四化学试剂厂, 氯乙酰氯:天津市密欧化学试剂有限公司, 氢氧化钾:天津市化学试剂供销公司, 盐酸:天津市永大化学试剂有限公司, 无水乙醇:天津市化学试剂供销公司, 1, 4-二氧六环:西陇化工有限公司.本实验分离纯化所用有机溶剂均为工业级, 经重新蒸馏后使用, 其他试剂均为市售分析纯, 必要时做常规处理.
3.2 实验方法
3.2.1 中间体的合成
化合物9a和9b按照文献[16, 17]的方法制备, 化合物9a和9b按照文献[16, 17]的方法制备, 化合物9a和9b按照文献[16, 17]的方法制备, 化合物15按照文献[16, 17]的方法制备.
3.2.2 目标化合物12的合成
将化合物11a (0.212 g, 0.60 mmol)和无取代的氨基查尔酮(0.110 g, 0.50 mmol)分别溶解于8 mL, 2 mL热的无水乙醇中, 升温至90 ℃, 将后者缓慢滴加入加热搅拌的前者体系中, 薄层色谱法监测反应, 待反应结束后停止反应, 冷却至室温后抽滤, 滤饼用分析纯的无水乙醇洗, 经真空干燥得黄色粉末状固体N-(苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-苯基嘧啶-4-胺(12a), 0.157 g, 收率64.5%. m.p. 178~179 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.73 (s, 1H), 8.18 (d, J=8.8 Hz, 2H), 8.01 (d, J=15.6 Hz, 1H), 7.95~7.92 (m, 2H), 7.83 (dd, J=5.1, 3.5 Hz, 4H), 7.77~7.72 (m, 4H), 7.48 (dd, J=5.5, 3.6 Hz, 8H), 7.26 (s, 1H), 5.05 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 187.20, 167.74, 161.57, 160.63, 151.75, 144.01, 143.13, 135.64, 134.82, 131.36, 131.08, 131.03, 130.79, 130.44, 130.05, 129.94, 129.16, 128.90, 128.86, 128.81, 128.32, 128.30, 126.66, 126.43, 126.33, 126.03, 125.69, 121.99, 118.71, 113.72, 99.93, 26.34; HR-MS (ESI) calcd for C33H26N5OS [M+H]+ 540.1858, found 540.1859.
化合物12b~12o的制备方法及投料比同化合物12a.
N-(2-氟苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-苯基嘧啶-4-胺(12b):黄色粉末状固体, 0.151 g, 产率55.1%. m.p. 190~191 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.78 (s, 1H), 8.19 (dd, J=15.0, 8.3 Hz, 3H), 8.05 (d, J=15.7 Hz, 1H), 7.87~7.82 (m, 5H), 7.74 (dd, J=6.2, 3.2 Hz, 2H), 7.48 (td, J=7.3, 4.1 Hz, 6H), 7.35 (dd, J=13.1, 5.7 Hz, 2H), 7.28 (s, 1H), 5.05 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 186.90, 167.68, 162.04, 161.53, 160.55, 159.55, 151.70, 144.14, 135.57, 134.14, 132.37, 130.99, 130.72, 129.95, 128.96, 128.80, 126.36, 125.63, 124.90, 123.99, 122.42, 122.31, 118.65, 116.09, 115.88, 113.66, 99.91, 26.28; HR-MS (ESI) calcd for C33H25FN5OS [M+H]+ 558.1764, found 558.1762.
N-(3-氟苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-苯基嘧啶-4-胺(12c):黄色粉末状固体, 0.059 g, 产率30.2%. m.p. 191~192 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.62 (s, 1H), 8.20 (d, J=8.8 Hz, 2H), 8.06 (d, J=15.6 Hz, 1H), 7.90 (d, J=10.2 Hz, 1H), 7.84 (d, J=8.4 Hz, 3H), 7.77~7.70 (m, 4H), 7.56~7.43 (m, 5H), 7.31 (td, J=8.5, 2.3 Hz, 1H), 7.22 (s, 1H), 5.02 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 187.02, 167.75, 163.65, 161.61, 161.23, 160.55, 151.74, 144.11, 141.57, 137.40, 137.32, 135.65, 131.12, 131.00, 130.79, 130.71, 130.00, 128.79, 126.36, 125.51, 125.44, 123.38, 118.63, 117.13, 116.92, 114.64, 114.42, 113.70, 99.88, 26.36; HR-MS (ESI) calcd for C33H25FN5O2S [M+H]+ 558.1764, found 558.1763.
N-(4-氟苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-苯基嘧啶-4-胺(12d):黄色粉末状固体, 0.072 g, 产率42.1%. m.p. 196~197 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.75 (s, 1H), 8.18 (d, J=8.8 Hz, 2H), 8.02 (dd, J=8.6, 5.7 Hz, 2H), 7.96 (s, 1H), 7.83 (dd, J=5.1, 3.5 Hz, 4H), 7.77~7.72 (m, 3H), 7.48 (td, J=7.3, 4.3 Hz, 5H), 7.33 (t, J=8.8 Hz, 2H), 7.27 (s, 1H), 5.05 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 187.13, 167.76, 164.55, 162.07, 161.67, 160.62, 151.80, 143.99, 141.91, 135.68, 131.53, 131.37, 131.19, 131.10, 130.80, 129.95, 128.88, 126.43, 125.72, 121.91, 118.73, 116.02, 115.80, 113.74, 99.93, 26.34; HR-MS (ESI) calcd for C33H25FN5OS [M+H]+ 558.1764, found 558.1763.
N-(3, 4-二氟苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-苯基嘧啶-4-胺(12e):黄色粉末状固体, 0.092 g, 产率50.6%. m.p. 172~174 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.74 (s, 1H), 8.19 (t, J=9.7 Hz, 3H), 8.05 (d, J=15.6 Hz, 1H), 7.84 (dd, J=7.4, 4.3 Hz, 4H), 7.73 (t, J=11.3 Hz, 4H), 7.59~7.45 (m, 7H), 7.26 (s, 1H), 5.04 (d, J=7.9 Hz, 2H); 13C NMR (100 MHz, DMSO-d6) δ: 196.57, 167.79, 163.87, 161.65, 161.42, 161.29, 160.52, 151.75, 144.23, 140.39, 138.62, 135.65, 131.21, 130.97, 130.74, 130.10, 128.81, 126.36, 125.42, 124.64, 118.62, 113.73, 112.72, 111.76, 111.50, 105.31, 99.90, 25.23; HR-MS (ESI) calcd for C33H24F2N5OS [M+H]+ 576.1760, found 576.1671.
N-(3, 5-二氟苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-苯基嘧啶-4-胺(12f):黄色粉末状固体, 0.158 g, 产率56.6%. m.p. 222~223 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.68 (s, 1H), 8.22 (d, J=8.8 Hz, 2H), 8.12 (d, J=15.6 Hz, 1H), 7.88~7.82 (m, 4H), 7.78~7.68 (m, 5H), 7.51~7.44 (m, 5H), 7.35 (dd, J=10.3, 8.1 Hz, 1H), 7.24 (s, 1H), 5.03 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 186.89, 167.79, 163.87, 161.65, 161.42, 161.29, 160.52, 151.75, 144.23, 140.39, 138.62, 135.65, 131.21, 130.97, 130.74, 130.10, 128.81, 126.36, 125.42, 124.64, 118.62, 113.73, 112.72, 111.76, 111.50, 105.31, 99.90, 26.40; HR-MS (ESI) calcd for C33H24F2N5O2S [M+H]+ 576.1760, found 576.1769.
N-(4-氯苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-苯基嘧啶-4-胺(12g):黄色粉末状固体, 0.089 g, 收率47.6%. m.p. 209~210 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.72 (s, 1H), 8.19 (d, J=8.8 Hz, 2H), 8.01 (dd, J=20.4, 12.1 Hz, 3H), 7.83 (d, J=8.6 Hz, 4H), 7.75~7.71 (m, 3H), 7.56 (d, J=8.5 Hz, 2H), 7.51~7.45 (m, 5H), 7.26 (s, 1H), 5.05 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 187.08, 167.73, 161.59, 160.62, 151.74, 144.09, 141.63, 135.64, 134.89, 133.81, 131.25, 130.79, 130.51, 129.98, 128.91, 128.86, 126.43, 125.70, 122.76, 118.71, 113.73, 99.94, 26.33; HR-MS (ESI) calcd for C33H25ClN5OS [M+H]+ 574.1668, found 574.1667.
N-(3, 5-二氯苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-苯基嘧啶-4-胺(12h):黄色粉末状固体, 0.113 g, 产率52.2%. m.p. 253~254 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.72 (s, 1H), 8.22 (d, J=8.8 Hz, 2H), 8.15 (d, J=15.6 Hz, 1H), 8.08 (d, J=1.6 Hz, 2H), 7.88~7.82 (m, 5H), 7.73 (dd, J=6.2, 3.2 Hz, 2H), 7.70 (s, 1H), 7.68~7.66 (m, 1H), 7.49~7.45 (m, 5H), 7.25 (s, 1H), 5.03 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 191.45, 186.89, 167.83, 161.72, 160.58, 151.84, 144.31, 139.96, 138.80, 138.59, 135.71, 134.60, 133.78, 131.19, 131.04, 130.80, 130.20, 129.22, 128.86, 127.56, 127.17, 126.43, 125.54, 124.88, 118.68, 113.80, 99.98, 26.44; HR-MS (ESI) calcd for C33H24Cl2N5OS [M+H]+ 608.1078, found 608.1077.
N-(2-溴苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-苯基嘧啶-4-胺(12i):黄色粉末状固体, 0.108 g, 产率46.0%. m.p. 198~199 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.83 (s, 1H), 8.30~8.26 (m, 1H), 8.20 (d, J=8.8 Hz, 2H), 8.02 (d, J=1.6 Hz, 2H), 7.85 (d, J=8.4 Hz, 4H), 7.79~7.71 (m, 4H), 7.51~7.45 (m, 6H), 7.30 (s, 1H), 5.05 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 186.94, 167.76, 161.65, 160.62, 151.76, 144.25, 140.43, 135.65, 134.07, 133.27, 132.03, 131.03, 130.79, 130.10, 128.87, 128.75, 128.23, 126.44, 125.71, 125.31, 124.87, 118.72, 113.73, 99.98, 26.34; HR-MS (ESI) calcd for C33H25BrN5OS [M+H]+618.0963, found 618.0966.
N-(3-溴苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-苯基嘧啶-4-胺(12j):黄色粉末状固体, 0.116 g, 产率50.7%. m.p. 204~205 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.70 (s, 1H), 8.25 (s, 1H), 8.21 (d, J=8.7 Hz, 2H), 8.08 (d, J=15.6 Hz, 1H), 7.90 (d, J=7.7 Hz, 1H), 7.85 (dd, J=7.7, 3.4 Hz, 4H), 7.73 (dd, J=6.0, 2.9 Hz, 3H), 7.70~7.61 (m, 2H), 7.49~7.44 (m, 6H), 7.25 (s, 1H), 5.03 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 192.00, 187.03, 167.82, 161.69, 160.61, 151.82, 144.19, 141.36, 137.67, 137.35, 135.71, 132.87, 131.54, 131.19, 131.12, 130.92, 130.79, 130.73, 130.33, 130.09, 128.86, 128.17, 126.43, 125.56, 123.48, 122.40, 121.44, 118.68, 113.78, 99.95, 26.42; HR-MS (ESI) calcd for C33H25BrN5OS [M+H]+ 618.0963, found 618.0963.
N-(4-溴苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-苯基嘧啶-4-胺(12k):黄色粉末状固体, 0.153 g, 产率60.2%. m.p. 229~230 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.68 (s, 1H), 8.18 (d, J=8.8 Hz, 2H), 8.04 (d, J=15.7 Hz, 1H), 7.90 (d, J=8.5 Hz, 2H), 7.86~7.82 (m, 4H), 7.77~7.67 (m, 6H), 7.47 (dd, J=6.3, 3.0 Hz, 6H), 7.24 (s, 1H), 5.03 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 187.10, 167.83, 161.72, 160.60, 151.79, 144.08, 141.74, 135.73, 134.13, 131.85, 131.27, 131.14, 130.78, 130.70, 129.99, 128.86, 126.43, 125.56, 123.76, 122.80, 118.72, 113.78, 99.93, 26.43; HR-MS (ESI) calcd for C33H25BrN5OS [M+H]+ 618.0963, found 618.0963.
N-(3, 5-二甲氧基苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-苯基嘧啶-4-胺(12l):制备方法及投料比同上, 得黄色粉末状固体化合物(0.100 g, 43.1%). m.p. 207~208 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.69 (s, 1H), 8.15 (d, J=8.8 Hz, 2H), 7.97 (d, J=9.7 Hz, 2H), 7.84 (t, J=7.1 Hz, 4H), 7.73 (dd, J=6.1, 3.1 Hz, 2H), 7.68 (dd, J=6.5, 2.9 Hz, 1H), 7.51~7.45 (m, 5H), 7.25 (s, 1H), 7.21~7.16 (m, 2H), 5.03 (d, J=8.0 Hz, 2H), 3.84 (d, J=14.9 Hz, 6H); 13C NMR (101 MHz, DMSO-d6)δ: 187.28, 167.79, 161.66, 160.62, 152.75, 151.81, 148.17, 144.02, 137.10, 135.70, 131.39, 130.95, 130.77, 129.90, 128.85, 128.35, 126.42, 125.64, 124.31, 122.79, 119.08, 118.72, 114.82, 113.75, 99.92, 60.98, 60.26, 55.79, 26.39; HR-MS (ESI) calcd for C35H30N5O3S [M+H]+ 600.2069, found 600.2068.
N-(4-羟基苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-苯基嘧啶-4-胺(12m):黄色粉末状固体, 0.080 g, 产率收率37.5%. m.p. 182~183 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.73 (d, J=3.7 Hz, 1H), 8.14 (d, J=8.8 Hz, 1H), 7.92 (d, J=8.8 Hz, 1H), 7.87~7.72 (m, 9H), 7.67 (d, J=15.4 Hz, 1H), 7.53~7.44 (m, 6H), 7.27 (d, J=2.3 Hz, 1H), 6.89 (d, J=8.6 Hz, 1H), 5.04 (d, J=6.7 Hz, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 196.29, 187.10, 167.73, 161.55, 160.64, 160.11, 151.76, 143.86, 143.67, 135.65, 131.80, 130.85, 130.78, 129.68, 129.45, 128.86, 126.43, 125.85, 125.71, 118.71, 118.60, 118.35, 115.84, 113.74, 99.84, 26.41, 26.29; HR-MS (ESI) calcd for C33H26N5O2S [M+H]+ 556.1807, found 556.1808.
N-(3-硝基苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-苯基嘧啶-4-胺(12n):黄色粉末状固体, 0.105 g, 产率44.9%. m.p. 194~195 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.63 (s, 1H), 8.81 (s, 1H), 8.39 (d, J=7.8 Hz, 1H), 8.30~8.27 (m, 1H), 8.25~8.21 (m, 3H), 8.19 (s, 1H), 7.92~7.80 (m, 7H), 7.79 (d, J=8.0 Hz, 1H), 7.76~7.72 (m, 3H), 7.53~7.43 (m, 7H), 7.22 (s, 1H), 5.02 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 186.92, 167.77, 161.63, 160.52, 151.76, 148.32, 144.22, 140.53, 136.67, 135.64, 134.93, 131.14, 130.98, 130.72, 130.25, 130.12, 128.79, 126.35, 125.47, 124.64, 124.44, 122.87, 118.61, 113.73, 99.89, 26.38; HR-MS (ESI) calcd for C33H25N6O3S [M+H]+ 585.1709, found 585.1710.
N-(2-噻吩苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-苯基嘧啶-4-胺(12o):黄色粉末状固体, 0.091 g, 产率40.8%. m.p. 204~205 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 11.31 (s, 1H), 10.81 (s, 1H), 9.91 (s, 1H), 8.28 (d, J=4.3 Hz, 1H), 8.03 (t, J=9.4 Hz, 3H), 7.93 (t, J=7.7 Hz, 1H), 7.63 (dd, J=8.1, 4.6 Hz, 3H), 7.51 (d, J=6.5 Hz, 3H), 7.14 (t, J=8.4 Hz, 3H), 7.09 (s, 1H), 4.25 (s, 2H); 13C NMR (100 MHz, DMSO-d6) δ: 186.67, 167.88, 161.75, 160.61, 151.85, 143.91, 139.87, 135.95, 135.74, 132.59, 131.32, 131.16, 130.80, 130.19, 129.75, 128.88, 128.70, 126.43, 125.58, 120.25, 118.76, 113.79, 99.91, 26.44; HR-MS (ESI) calcd for C31H24N5OS2 [M+H]+ 546.1422, found 546.1423.
3.2.3 目标化合物13的合成
将化合物11b (0.174 g, 0.60 mmol)和无取代的氨基查尔酮(0.157 g, 0.50 mmol)分别溶解于8 mL, 2 mL无水乙醇中, 升温至90 ℃, 将后者缓慢滴加入加热搅拌的前者体系中, 薄层色谱法监测反应, 待反应结束后停止反应, 冷却至室温后抽滤, 滤饼用分析纯的无水乙醇洗涤, 真空干燥得黄色粉末状固体N-(苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13a), 0.067 g, 收率56.6%. m.p. 177~178 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.65 (s, 1H), 8.12 (d, J=8.7 Hz, 2H), 8.04~7.91 (m, 3H), 7.78~7.64 (m, 5H), 7.48 (t, J=4.7 Hz, 5H), 6.63 (s, 1H), 4.97 (s, 2H), 2.27 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 187.79, 166.24, 160.33, 151.09, 143.86, 143.25, 135.27, 132.67, 131.21, 131.00, 130.23, 129.41, 129.37, 126.16, 122.43, 120.21, 114.21, 103.02, 26.54, 21.99; HR-MS (ESI) calcd for C28H24N5OS [M+ H]+ 478.1701, found 478.1702.
化合物13b~13y的制备方法及投料比同化合物13a.
N-(2-氟苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13b):黄色粉末状固体, 0.120 g, 收率48.4%. m.p. 178~179 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.81 (s, 1H), 8.21 (t, J=7.6 Hz, 1H), 8.06 (dd, J=25.3, 12.2 Hz, 2H), 7.87~7.79 (m, 1H), 7.75~7.63 (m, 4H), 7.61~7.43 (m, 4H), 7.35 (dd, J=13.1, 5.6 Hz, 2H), 6.65 (d, J=7.6 Hz, 1H), 4.98 (s, 2H), 2.27 (d, J=5.0 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 187.54, 166.79, 164.34, 162.62, 160.38, 160.12, 151.56, 143.94, 134.85, 133.11, 131.98, 131.22, 130.36, 129.55, 126.21, 125.51, 124.50, 122.92, 122.81, 119.79, 116.68, 116.46, 114.23, 103.21, 26.53, 22.79; HR-MS (ESI) calcd for C28H23FN5OS [M+H]+ 496.1607, found 496.1607.
N-(3-氟苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13c):黄色粉末状固体, 0.109 g, 收率55.1%. m.p. 161~162 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.65 (s, 1H), 8.12 (d, J=8.7 Hz, 2H), 8.04~7.91 (m, 3H), 7.78~7.64 (m, 5H), 7.48 (t, J=4.7 Hz, 5H), 6.63 (s, 1H), 4.97 (s, 2H), 2.27 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 187.49, 166.58, 160.37, 151.31, 143.83, 141.06, 134.54, 133.76, 132.56, 132.09, 131.24, 130.42, 129.31, 128.74, 126.17, 125.82, 125.33, 119.95, 114.23, 103.16, 26.52, 22.48; HR-MS (ESI) calcd for C28H23FN5OS [M+H]+ 496.1607, found 496.1608.
N-(4-氟苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13d):黄色粉末状固体, 0.032 g, 收率26.9%. m.p. 171~172 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.62 (s, 1H), 8.12 (d, J=8.8 Hz, 2H), 8.06~7.92 (m, 3H), 7.77~7.66 (m, 5H), 7.48 (dd, J=6.1, 3.2 Hz, 2H), 7.33 (t, J=8.8 Hz, 2H), 6.61 (d, J=5.7 Hz, 1H), 4.96 (s, 2H), 2.26 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 187.69, 166.26, 165.07, 162.59, 160.33, 151.09, 143.28, 142.60, 132.62, 131.98, 131.95, 131.76, 131.68, 131.21, 130.23, 126.16, 122.34, 120.17, 116.51, 116.29, 114.89, 114.21, 103.03, 55.86, 26.53, 22.03; HR-MS (ESI) calcd for C28H23FN5OS [M+H]+ 496.1607, found 496.1605.
N-(3, 4-二氟苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13e):黄色粉末状固体, 0.194 g, 收率37.9%. m.p. 165~166 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.65 (s, 1H), 8.12 (d, J=8.7 Hz, 2H), 8.04~7.91 (m, 3H), 7.78~7.64 (m, 5H), 7.48 (t, J=4.7 Hz, 5H), 6.63 (s, 1H), 4.97 (s, 2H), 2.27 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 187.55, 166.40, 160.34, 151.17, 143.56, 142.73, 141.49, 133.29, 132.33, 131.22, 130.35, 130.20, 127.26, 126.16, 123.73, 121.36, 120.05, 118.52, 118.34, 117.61, 117.44, 103.07, 56.66, 26.52, 22.25; HR-MS (ESI) calcd for C28H22F2N5OS [M+H]+ 514.1513, found 514.1511.
N-(3, 5-二氟苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13f):黄色粉末状固体, 0.194 g, 收率37.9%. m.p. 182~183 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.73 (s, 1H), 8.14 (dd, J=14.4, 12.3 Hz, 3H), 7.95~7.66 (m, 7H), 7.48 (dd, J=6.2, 3.2 Hz, 2H), 7.41~7.30 (m, 1H), 6.64 (s, 1H), 4.97 (s, 2H), 2.27 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 187.54, 166.47, 164.42, 164.29, 161.98, 161.84, 160.34, 151.25, 143.78, 141.12, 139.13, 132.12, 131.21, 130.47, 129.17, 128.91, 126.16, 125.17, 119.98, 114.21, 112.38, 112.12, 105.90, 103.12, 26.52, 22.35; HR-MS (ESI) calcd for C28H22F2N5OS [M+H]+ 514.1513, found 514.1509.
N-(4-氯苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13g):黄色粉末状固体, 0.058 g, 收率47.2%. m.p. 176~177 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.47 (s, 1H), 8.12 (d, J=8.8 Hz, 2H), 7.99 (dd, J=12.0, 9.9 Hz, 3H), 7.76~7.66 (m, 5H), 7.56 (d, J=8.5 Hz, 2H), 7.52~7.46 (m, 2H), 6.59 (s, 1H), 4.94 (s, 2H), 2.25 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 187.66, 166.16, 162.67, 160.30, 151.01, 143.26, 142.37, 135.45, 134.25, 132.60, 131.20, 131.07, 130.26, 129.42, 126.17, 123.18, 120.25, 114.20, 103.04, 26.54, 21.89; HR-MS (ESI) calcd for C28H23ClN5OS [M+H]+ 512.1312, found 512.1313.
N-(2, 4-二氯苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13h):黄色粉末状固体, 0.120 g, 收率54.8%. m.p. 169~170 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.74 (s, 1H), 8.36 (d, J=8.6 Hz, 1H), 8.11 (dd, J=19.9, 12.1 Hz, 3H), 7.96 (d, J=15.5 Hz, 1H), 7.82~7.65 (m, 5H), 7.58 (dd, J=8.5, 1.8 Hz, 1H), 7.48 (dd, J=6.1, 3.1 Hz, 2H), 6.65 (s, 1H), 4.97 (s, 2H), 2.27 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 187.35, 166.49, 163.55, 160.34, 151.25, 143.82, 137.05, 135.96, 135.56, 132.05, 131.95, 131.22, 130.45, 130.37, 129.94, 128.42, 126.18, 125.73, 119.99, 114.23, 103.16, 26.52, 22.37; HR-MS (ESI) calcd for C28H22Cl2N5OS [M+H]+ 546.0922, found 546.0921.
N-(2, 5-二氯苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13i):黄色粉末状固体, 0.138 g, 收率24.9%. m.p. 174~175 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.73 (s, 1H), 7.91 (d, J=8.7 Hz, 2H), 7.84 (d, J=6.7 Hz, 2H), 7.78~7.71 (m, 4H), 7.53~7.42 (m, 5H), 7.27 (s, 1H), 5.03 (s, 2H), 2.54 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 196.8, 168.2, 161.8, 161.1, 152.2, 144.3, 136.0, 131.3, 129.9, 129.3, 126.9, 126.2, 119.1, 114.2, 100.4, 26.9, 26.8; HR-MS (ESI) calcd for C28H22Cl2N5OS [M+H]+ 554.2014, found 554.2014.
N-(3, 5-二氯苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13j):黄色粉末状固体, 0.153 g, 收率56.0%). m.p. 182~183 ℃; 1H NMR (400 MHz, DMSO-d6 δ: 10.64 (s, 1H), 8.15 (dd, J=12.1, 10.0 Hz, 3H), 8.09 (d, J=1.8 Hz, 2H), 7.80~7.64 (m, 6H), 7.49 (dd, J=6.2, 3.1 Hz, 2H), 6.62 (s, 1H), 4.94 (d, J=5.5 Hz, 2H), 2.25 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 169.3, 161.8, 161.2, 159.5, 157.2, 153.6, 139.6, 136.8, 136.1, 131.0, 129.3, 127.0, 123.0, 122.6, 116.2, 115.9, 115.8, 115.0, 98.7, 28.4; HR-MS (ESI) calcd for C28H22Cl2N5OS [M+H]+ 546.0922, found 546.0923.
N-(2-溴苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13k):黄色粉末状固体, 0.070 g, 收率31.4%. m.p. 170~171 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.65 (s, 1H), 8.12 (d, J=8.7 Hz, 2H), 8.04~7.91 (m, 3H), 7.78~7.64 (m, 5H), 7.48 (t, J=4.7 Hz, 5H), 6.63 (s, 1H), 4.97 (s, 2H), 2.27 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 187.66, 166.63, 164.21, 161.79, 160.38, 151.39, 143.79, 142.31, 137.92, 137.84, 132.21, 131.37, 131.24, 130.39, 126.17, 126.01, 123.93, 119.89, 117.73, 117.51, 115.27, 115.06, 114.23, 103.11, 26.51, 22.59; HR-MS (ESI) calcd for C28H23BrN5OS [M+H]+ 556.0806, found 556.0825.
N-(3-溴苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13l):黄色粉末状固体, 0.103 g, 收率46.1%. m.p. 169~170 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.58 (s, 1H), 8.24 (s, 1H), 8.14 (d, J=8.8 Hz, 2H), 8.05 (d, J=15.6 Hz, 1H), 7.91 (d, J=7.8 Hz, 1H), 7.79~7.58 (m, 6H), 7.55~7.41 (m, 3H), 6.61 (s, 1H), 4.94 (d, J=7.9 Hz, 2H), 2.26 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 187.59, 166.64, 160.38, 151.44, 143.83, 142.01, 137.82, 133.41, 132.19, 131.43, 131.32, 131.24, 130.43, 128.65, 126.19, 123.95, 122.89, 119.86, 114.24, 103.13, 26.50, 22.62; HR-MS (ESI) calcd for C28H23BrN5OS [M+H]+ 556.0806, found 556.0806.
N-(4-溴苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13m):黄色粉末状固体, 0.110 g, 收率89.1%. m.p. 180~181 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.60 (s, 1H), 8.12 (d, J=8.7 Hz, 2H), 8.02 (d, J=15.6 Hz, 1H), 7.91 (d, J=8.5 Hz, 2H), 7.79~7.65 (m, 6H), 7.48 (dd, J=6.1, 3.2 Hz, 2H), 6.62 (s, 1H), 4.96 (s, 3H), 2.26 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 187.66, 166.18, 164.03, 163.57, 162.90, 161.25, 160.31, 151.03, 143.29, 142.45, 134.58, 132.57, 132.34, 131.28, 131.20, 130.27, 126.17, 124.33, 123.23, 120.23, 114.21, 103.03, 56.48, 26.54, 21.93, 19.03; HR-MS (ESI) calcd for C28H23BrN5OS [M+H]+ 556.0806, found 556.0803.
N-(2-氟-4-溴苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13n):黄色粉末状固体, 0.112 g, 收率63.2%. m.p. 171~172 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.86 (d, J=11.3 Hz, 1H), 8.06 (dd, J=31.4, 12.2 Hz, 2H), 7.91 (d, J=8.4 Hz, 1H), 7.82 (d, J=8.6 Hz, 1H), 7.74~7.65 (m, 4H), 7.56 (d, J=8.6 Hz, 1H), 7.48 (ddd, J=12.8, 6.1, 3.1 Hz, 2H), 6.66 (d, J=6.1 Hz, 1H), 4.97 (d, J=9.5 Hz, 2H), 2.28 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ: 168.4, 165.8, 162.2, 161.2, 153.7, 144.3, 136.2, 131.3, 130.7, 130.1, 129.4, 126.9, 126.1, 123.8, 119.3, 115.9, 114.2, 100.2, 60.8, 27.1, 14.7; HR-MS (ESI) calcd for C28H22BrFN5OS [M+H]+ 574.0712, found 574.0713.
N-(3-溴-4-氟苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13o):黄色粉末状固体, 0.289 g, 收率58.5%. m.p. 179~180 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 11.01 (s, 1H), 8.42 (d, J=6.7 Hz, 1H), 8.13 (d, J=8.6 Hz, 2H), 8.02 (dd, J=15.5, 11.0 Hz, 2H), 7.75~7.66 (m, 5H), 7.51~7.44 (m, 3H), 6.70 (d, J=5.0 Hz, 1H), 5.00 (s, 2H), 2.51 (d, J=1.5 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 187.03, 166.02, 163.20, 160.41, 159.87, 157.93, 150.81, 143.20, 140.61, 133.33, 131.78, 130.73, 130.61, 129.89, 129.28, 125.68, 123.18, 119.45, 117.24, 117.01, 113.73, 108.96, 108.75, 102.59, 26.45, 25.99, 21.95; HR-MS (ESI) calcd for C28H22BrFN5OS [M+H]+ 574.0712, found 574.0711.
N-(4-甲基苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13p):黄色粉末状固体, 0.125 g, 收率50.8%. m.p. 189~190 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.72 (s, 1H), 8.10 (d, J=8.6 Hz, 2H), 7.88 (dd, J=39.0, 11.8 Hz, 3H), 7.78~7.62 (m, 5H), 7.48 (dd, J=6.1, 3.1 Hz, 2H), 7.30 (d, J=7.9 Hz, 2H), 6.64 (s, 1H), 4.97 (s, 2H), 2.37 (s, 3H), 2.27 (d, J=5.4 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 187.6, 166.00, 162.19, 160.29, 150.90, 143.95, 142.93, 141.05, 132.95, 132.54, 131.20, 130.12, 130.03, 129.41, 126.15, 121.34, 120.37, 114.20, 102.96, 99.99, 26.55, 21.58; HR-MS (ESI) calcd for C29H26N5OS [M+H]+ 492.1858, found 492.1856.
N-(4-异丙基苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13q):黄色粉末状固体, 0.058 g, 收率45.0%. m.p. 186~187 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.58 (d, J=14.4 Hz, 1H), 8.10 (d, J=8.8 Hz, 1H), 7.92 (d, J=15.6 Hz, 1H), 7.88~7.82 (m, 2H), 7.82~7.61 (m, 5H), 7.60~7.47 (m, 3H), 7.36 (d, J=8.2 Hz, 1H), 6.61 (d, J=6.3 Hz, 1H), 4.95 (d, J=10.6 Hz, 2H), 4.86 (s, 1H), 2.26 (d, J=3.2 Hz, 3H), 2.10 (s, 1H), 1.92 (s, 3H), 1.24 (d, J=6.9 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ: 187.75, 166.56, 160.37, 151.81, 151.34, 143.88, 143.46, 132.98, 132.55, 131.23, 130.19, 129.52, 127.40, 126.17, 121.46, 119.95, 114.22, 103.08, 33.90, 26.53, 24.11, 22.46; HR-MS (ESI) calcd for C31H30N5OS [M+H]+ 520.2171, found 520.2173.
N-(4-叔丁基苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13r):黄色粉末状固体, 0.035 g, 收率27.3%. m.p. 182~183 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.64 (s, 1H), 8.10 (d, J=8.8 Hz, 2H), 7.89 (dd, J=27.1, 12.0 Hz, 3H), 7.70 (ddd, J=20.5, 11.0, 5.5 Hz, 5H), 7.56~7.42 (m, 4H), 6.63 (s, 1H), 4.97 (s, 2H), 2.27 (s, 3H), 1.32 (s, 9H); 13C NMR (100 MHz, DMSO-d6) δ: 171.2, 171.1, 165.6, 161.6, 151.2, 150.8, 148.6, 139.1, 134.8, 132.6, 129.4, 128.0, 120.1, 113.6, 113.2, 36.2; HR-MS (ESI) calcd for C32H31N5OS [M+H]+ 534.2327, found 534.2330.
N-(3-羟基苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13s):黄色粉末状固体, 0.086 g, 收率56.7%. m.p. 194~195 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.80 (s, 1H), 7.86 (d, J=8.6 Hz, 2H), 7.62 (s, 1H), 7.51 (dd, J=6.1, 3.2 Hz, 3H), 7.44 (dd, J=12.2, 5.3 Hz, 3H), 7.29~7.25 (m, 2H), 7.16 (d, J=7.7 Hz, 1H), 7.08 (dd, J=9.7, 5.0 Hz, 2H), 6.73 (dd, J=7.9, 2.0 Hz, 1H), 6.51 (s, 1H), 4.80 (s, 2H), 2.32 (d, J=1.7 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 187.31, 165.96, 163.02, 159.88, 157.80, 150.83, 143.62, 142.92, 135.99, 132.09, 130.73, 129.84, 129.68, 129.28, 125.70, 121.72, 119.67, 119.57, 117.73, 115.31, 113.74, 102.55, 55.98, 26.01, 21.84, 18.52; HR-MS (ESI) calcd for C28H24N5O2S [M+H]+ 494.1650, found 494.1650.
N-(2, 3-二甲氧基苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13t):黄色粉末状固体, 0.097 g, 收率61.0%. m.p. 180~181 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.70 (s, 1H), 8.09 (d, J=8.8 Hz, 2H), 7.96 (q, J=15.7 Hz, 2H), 7.70 (ddd, J=19.3, 9.4, 6.0 Hz, 5H), 7.48 (dd, J=6.2, 3.2 Hz, 2H), 7.21~7.16 (m, 2H), 6.64 (s, 1H), 4.97 (s, 2H), 3.86 (s, 3H), 3.82 (s, 3H), 2.26 (d, J=5.9 Hz, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 168.1, 161.9, 161.1, 153.7, 140.7, 136.1, 133.3, 131.3, 131.1, 130.3, 130.0, 129.9, 129.7, 129.2, 126.8, 125.9, 123.6, 123.2, 119.2, 116.2, 115.9, 114.1, 99.8, 26.9; HR-MS (ESI) calcd for C30H28N5O3S [M+H]+ 538.1913, found 538.1915.
N-(3, 4, 5-三甲氧苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13u):黄色粉末状固体, 0.157 g, 收率64.4%. m.p. 183~184 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.36 (s, 1H), 8.18 (d, J=8.6 Hz, 2H), 7.97 (d, J=15.5 Hz, 1H), 7.71 (dd, J=10.9, 8.1 Hz, 5H), 7.49 (dd, J=6.0, 3.0 Hz, 2H), 7.28 (s, 2H), 6.55 (s, 1H), 4.93 (s, 2H), 3.90 (s, 6H), 3.72 (s, 3H), 2.24 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 187.65, 166.92, 164.63, 160.41, 153.61, 151.58, 144.23, 144.02, 143.80, 143.46, 140.11, 132.27, 131.26, 130.89, 130.39, 126.16, 121.64, 119.58, 114.21, 107.01, 103.17, 60.61, 56.73, 26.54, 22.98; HR-MS (ESI) calcd for C31H30N5O4S [M+H]+ 568.2018, found 568.2016.
N-(3-甲氧基4-羟基二氟苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13v): 黄色粉末状固体, 0.074 g, 收率28.2%. m.p. 179~180 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.70 (s, 1H), 8.09 (d, J=8.8 Hz, 2H), 7.96 (q, J=15.7 Hz, 2H), 7.70 (ddd, J=19.3, 9.4, 6.0 Hz, 5H), 7.48 (dd, J=6.2, 3.2 Hz, 2H), 7.21~7.16 (m, 2H), 6.64 (s, 1H), 4.97 (s, 2H), 3.86 (s, 3H), 3.82 (s, 3H), 2.26 (d, J=5.9 Hz, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 196.82, 166.25, 162.95, 160.37, 151.14, 143.17, 132.06, 131.22, 130.68, 129.77, 126.20, 120.11, 114.26, 102.89, 99.99, 26.98, 26.45, 22.04; HR-MS (ESI) calcd for C29H26N5O3S [M+H]+ 524.1756, found 524.1758.
N-(3-硝基苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13w):黄色粉末状固体, 0.176 g, 收率41.9%. m.p. 170~171 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.65 (s, 1H), 8.79 (s, 1H), 8.41 (d, J=7.8 Hz, 1H), 8.29 (dd, J=8.2, 1.5 Hz, 1H), 8.18 (dd, J=12.2, 9.0 Hz, 3H), 7.90~7.67 (m, 6H), 7.49 (dd, J=6.2, 3.2 Hz, 2H), 6.63 (s, 1H), 4.96 (s, 2H), 2.26 (s, 3H); 13C NMR (101 MHz, DMSO-d6) δ: 187.58, 166.65, 160.38, 151.43, 148.90, 143.94, 141.23, 137.20, 135.42, 132.07, 131.25, 130.84, 130.51, 126.19, 125.22, 125.05, 123.55, 119.87, 114.25, 103.12, 26.49, 22.62; HR-MS (ESI) calcd for C28H23N6O3S [M+H]+ 523.1552, found 523.1551.
N-(2-噻吩苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13x):黄色粉末状固体, 0.082 g, 收率33.9%. m.p. 190~191 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.73 (s, 1H), 8.02 (d, J=8.8 Hz, 2H), 7.90 (d, J=15.3 Hz, 1H), 7.81 (d, J=5.0 Hz, 1H), 7.73 (dd, J=6.0, 3.2 Hz, 3H), 7.64 (d, J=8.8 Hz, 2H), 7.59 (d, J=15.3 Hz, 1H), 7.49 (dd, J=6.1, 3.2 Hz, 2H), 6.65 (s, 1H), 4.97 (s, 2H), 2.27 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ: 189.50, 166.56, 160.37, 151.81, 151.34, 143.88, 143.46, 132.98, 132.55, 131.23, 130.19, 129.52, 127.40, 126.17, 121.46, 119.95, 114.22, 103.08, 33.90, 26.53, 24.11, 20.31; HR-MS (ESI) calcd for C26H22N5OS2 [M+H]+ 484.1266, found 484.1266.
N-(2-呋喃苯乙烯基苯基酮)-2-(苯并咪唑-2-亚甲硫基)-6-甲基嘧啶-4-胺(13y):黄色粉末状固体, 0.155 g, 收率66.2%. m.p. 186~187 ℃; 1H NMR (400 MHz, DMSO-d6) δ: 10.68 (s, 1H), 7.97 (dd, J=5.2, 3.5 Hz, 3H), 7.73 (dd, J=6.2, 3.2 Hz, 2H), 7.63 (d, J=8.8 Hz, 2H), 7.56 (s, 2H), 7.50 (dd, J=6.2, 3.2 Hz, 2H), 7.14 (d, J=3.3 Hz, 1H), 6.72 (dd, J=3.4, 1.8 Hz, 1H), 6.63 (s, 1H), 4.96 (s, 2H), 2.27 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ: 186.69, 165.99, 159.89, 151.26, 150.84, 146.09, 142.93, 131.98, 130.76, 129.88, 129.45, 125.73, 119.58, 118.65, 116.69, 113.76, 113.10, 102.55, 26.01, 21.83.; HR-MS (ESI) calcd for C26H22N5O2S [M+H]+ 468.1494, found 468.1494.
3.2.4 抗肿瘤细胞毒活性实验
初步筛选:取对数生长期的细胞, 消化、计数, 以6000个/孔的细胞密度接种至96孔板中, 每孔100 μL, 培养24 h后, 弃去培养基, 加入提前用培养基稀释好的样品, 每个孔200 μL(浓度梯度为50和100 μg/mL), 每个浓度设6个复孔另设空白对照组及阴性对照组.药物作用72 h后, 每孔加入5 μL CCK-8进行染色, 继续培养4 h后, 加入150 μL的二甲基亚砜, 振荡均匀, 酶标仪450 nm处检测吸光度值, 计算抑制率.进一步筛选:样品抑制率大于50%的样品进行半数抑制率(IC50)实验.即将待测样品以100, 50, 25, 12.5, 6.25, 3.125, 1.5625, 0.78125 μg/mL浓度加入96孔板中, 实验方法同上, 培养72 h后, 测450 nm时的吸光度值, 通过SPSS16.0统计软件计算得出IC50值.
辅助材料(Supporting Information) 化合物12a~12o和13a~13y的1H NMR和13C NMR谱图.这些材料可以免费从本刊网站(http://sioc-journal.cn/)上下载.
-
-
[1]
For statistical information about cancer, see:U. S. Food and Drug Administration. http://www.fda.gov/AboutFDA/ReportsManuals-Forms/Reports/ucm276385.htm.
-
[2]
Sangthong, S.; Krusong, K.; Ngamrojanavanich, N.; Vilaivan, T.; Puthong, S.; Chandchawan, S.; Muangsin, N. Bioorg. Med. Chem. Lett. 2011, 21, 4813. doi: 10.1016/j.bmcl.2011.06.052
-
[3]
Cao, R. H.; Fan, W. X.; Guo, L.; Ma, Q.; Zhang, G. X.; Li, J. R.; Chen, X. M.; Ren, Z. H.; Qiu, L. Q. Eur. J. Med. Chem. 2013, 60, 135. doi: 10.1016/j.ejmech.2012.11.045
-
[4]
Kamal, A.; Tamboli, J. R.; Nayak, V. L.; Adil, S. F.; Vish-nuvardhan, M. V. P. S.; Ramakrishna, S. Bioorg. Med. Chem. Lett. 2013, 23, 3208. doi: 10.1016/j.bmcl.2013.03.129
-
[5]
Kamal, A.; Dastagiri, D.; Ramaiah, M. J.; Reddy, J. S.; Bharathi, E. V.; Reddy, M. K.; Sagar, M. V. P.; Reddy, T. L.; Pushpavalli, S. N. C. V. L.; Pal-Bhadra, M. Eur. J. Med. Chem. 2011, 46, 5817. doi: 10.1016/j.ejmech.2011.09.039
-
[6]
Hamid, M. K. A. E.; Mihovilovic, M. D.; El-Nassan, H. B. Eur. J. Med. Chem. 2012, 57, 323. doi: 10.1016/j.ejmech.2012.09.031
-
[7]
Kassab, A. E.; Gedawy, E. M. Eur. J. Med. Chem. 2013, 63, 224. doi: 10.1016/j.ejmech.2013.02.011
-
[8]
Duschinsky, R.; Pleven, E.; Heidelberger, C. J. Am. Chem. Soc. 1957, 79, 4559.
-
[9]
Pecchi, S.; Renhowe, P. A.; Taylor, C.; Kaufman, S.; Merritt, H.; Wiesmann, M.; Shoemaker, K. R.; Knapp, M.; Ornelas, E.; Hendrickson, T. F.; Fantl, W.; Voliva, C. F. Bioorg. Med. Chem. Lett. 2010, 20, 6895. doi: 10.1016/j.bmcl.2010.10.021
-
[10]
Keche, A. P.; Hatnapure, G. D.; Tale, R. H.; Rodge, A. H.; Bi-rajdar, S. S.; Kamble, V. M. Bioorg. Med. Chem. Lett. 2012, 22, 3445. doi: 10.1016/j.bmcl.2012.03.092
-
[11]
Ni, L.; Meng, C. Q.; Sikorski, J. A. Expert. Opin. Ther. Pat. 2004, 14, 1669. doi: 10.1517/13543776.14.12.1669
-
[12]
Dimmock, J. R.; Elias, D. W.; Beazely, M. A. Kandepu, N. M. Curr. Med. Chem. 1999, 6, 1125.
-
[13]
Domínguez, J. N.; León, C.; Rodrigues, J.; Gamboa, D. N.; Gut, J.; Rosenthal, P. J. J. Med. Chem. 2005, 48, 3654. doi: 10.1021/jm058208o
-
[14]
Gacche, R.; Dhole, N. A.; Kamble, S. G.; Bandgar, B. P. J. En-zyme Inhib. Med. Chem. 2008, 23, 28. doi: 10.1080/14756360701306370
-
[15]
Sharma, V.; Kumar, P.; Pathak, D. J. Heterocycl. Chem. 2010, 47, 491.
-
[16]
Shao, K. P.; Zhang, X. Yao.; Chen, P. J.; Xue, D. Q.; He, P.; Ma, L. Y.; Zheng, J. X.; Zhang, Q. R.; Liu, H. M. Bioorg. Med. Chem. Lett. 2014, 24, 3877. doi: 10.1016/j.bmcl.2014.06.050
-
[17]
Chen, P. J.; Yang, A.; Gu, Y. F.; Zhang, X. S.; Shao, K. P.; Xue, D. Q.; He, P.; Jiang, T. F.; Zhang, Q. R.; Liu, H. M. Bioorg. Med. Chem. Lett. 2014, 24, 2741. doi: 10.1016/j.bmcl.2014.04.037
-
[18]
Tominaga, H.; Ishiyama, M.; Ohseto, F.; Sasamoto, K.; Hamamoto, T.; Suzuki, K.; Watanabe, M. Anal. Commun. 1999, 36, 47. doi: 10.1039/a809656b
-
[1]
-
表 1 化合物12a~12o和13a~13y的抗肿瘤活性[IC50/(μmol•L-1)]a
Table 1. Antitumor activity [IC50/(μmol•L-1)] of target compounds 12a~12o and 13a~13y
Compd. R1 R2 MGC-803 HepG-2 EC-109 MDA-MB-231 12a Ph H >100 33.90±1.60 36.66±1.02 >100 12b Ph 2-F >100 >100 >100 >100 12c Ph 3-F >100 >100 >100 >100 12d Ph 4-F >100 20.39±1.67 55.37±1.65 >100 12e Ph 3, 4-F2 >100 >100 >100 10.89±0.22 12f Ph 3, 5-F2 21.67±0.87 27.69±0.63 18.70±0.94 4.11±0.34 12g Ph 4-Cl >100 >100 23.00±1.24 19.90±1.13 12h Ph 3, 5-Cl2 24.82±1.32 9.75±1.81 20.67±1.26 6.45±0.65 12i Ph 2-Br 65.83±1.65 76.35±1.85 >100 16.90±0.64 12j Ph 3-Br >100 44.50±1.36 >100 >100 12k Ph 4-Br >100 >100 >100 >100 12l Ph 3, 5-(OCH3)2 >100 >100 >100 >100 12m Ph 4-HO >100 >100 >100 >100 12n Ph 3-NO2 >100 >100 >100 >100 12o Ph 2-Thienyl >100 >100 >100 45.86±1.54 13a CH3 H >100 >100 >100 >100 13b CH3 2-F 40.12±1.36 31.46±1.14 44.50±0.90 19.99±0.65 13c CH3 3-F 5.45±1.85 20.67±1.38 19.24±1.35 8.86±1.27 13d CH3 4-F 7.01±0.09 9.92±0.36 10.87±0.99 9.99±0.86 13e CH3 3, 4-F2 13.42±0.50 13.41±0.52 19.80±1.20 15.00±0.71 13f CH3 3, 5-F2 7.15±1.43 8.37±0.97 14.41±0.94 2.63±0.46 13g CH3 4-Cl >100 >100 >100 33.08±0.58 13h CH3 2, 4-Cl2 9.85±0.96 33.33±0.33 40.29±0.69 8.68±0.08 13i CH3 2, 5-Cl2 >100 82.59±0.62 >100 31.03±0.55 13j CH3 3, 5-Cl2 25.45±1.11 >100 >100 >100 13k CH3 2-Br 19.14±1.11 6.80±1.05 25.90±1.22 2.83±0.88 13l CH3 3-Br 20.64±0.79 4.89±1.21 22.72±0.93 10.67±0.66 13m CH3 4-Br 3.19±0.07 10.05±0.06 11.99±1.19 12.46±0.66 13n CH3 2-F-4-Br 7.61±1.24 28.53±1.74 17.04±1.26 17.50±1.03 13o CH3 4-F-3-Br 2.90±1.36 15.94±1.35 23.17±1.37 6.69±0.76 13p CH3 4-CH3- 14.60±1.13 24.19±0.80 33.11±1.19 17.94±0.87 13q CH3 4-CH(CH3)2 41.71±1.07 35.54±1.23 50.21±1.36 48.73±0.12 13r CH3 4-C(CH3)3 15.95±1.84 36.41±1.88 25.00±0.83 10.91±0.08 13s CH3 3-HO 13.67±0.23 18.66±0.91 20.76±1.05 6.77±0.84 13t CH3 2, 3-(OCH3)2 10.62±0.95 18.33±1.31 58.80±1.52 25.85±1.43 13u CH3 3, 4, 5-(OCH3)3 0.99±0.19 21.19±0.86 20.43±1.04 1.77±0.27 13v CH3 4-OH-3-OCH3 37.88±1.40 >100 >100 >100 13w CH3 3-NO2 1.43±0.15 2.20±0.38 16.71±0.51 3.02±0.21 13x CH3 2-Thienyl 4.05±0.51 5.27±0.88 12.80±0.64 1.05±0.14 13y CH3 2-Furyl 3.49±0.69 13.05±0.85 23.67±1.30 2.14±0.21 5-Fub 7.96± 0.87 10.36±0.28 16.64±1.12 6.58±0.38 a Antitumor activity was assayed by exposure for 72 h to substances and expressed as concentration required to inhibit tumor cell proliferation by 50% (IC50). Dates are presented as the means±SDs of three independent experiments. b Positive control. -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 10
- 文章访问数: 2293
- HTML全文浏览量: 220


 下载:
下载:


 下载:
下载:

