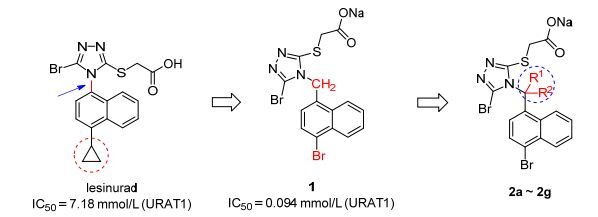
 图式1
本工作中URAT1抑制剂的设计思路
Scheme1.
Design of URAT1 inhibitors in present study
图式1
本工作中URAT1抑制剂的设计思路
Scheme1.
Design of URAT1 inhibitors in present study

Citation: Cai Wenqing, Liu Wei, Zhang Shuo, Wang Jianwu, Zhao Guilong. Design, Synthesis and Bioactivity of Highly Sterically Congested Flexible Uric Acid Transporter 1 (URAT1) Inhibitors[J]. Chinese Journal of Organic Chemistry, 2017, 37(9): 2303-2314. doi: 10.6023/cjoc201704038

含大位阻结构的柔性尿酸转运体1(URAT1) 抑制剂的设计、合成和生物活性研究
English
Design, Synthesis and Bioactivity of Highly Sterically Congested Flexible Uric Acid Transporter 1 (URAT1) Inhibitors
-
Key words:
- gout
- / hyperuricemia
- / URAT1 inhibitor
- / structure-activity relationship
- / lesinurad
- / steric congestion
-
痛风是世界上最古老的炎症性关节疾病之一[1], 系由尿酸单钠盐在关节或者其周围组织沉积所导致, 其特征是在病变部位反复发作的炎症性红肿和疼痛[2].高尿酸血症是痛风形成的前提条件, 是血液中尿酸单钠盐浓度高于其在生理温度和pH下最大溶解度(6.8 mg/dL, 404 μmol/L)的一种疾病[3].痛风的发病率在发达国家高于发展中国家, 但是在过去几十年里都呈现明显的上升趋势[4].尿酸是嘌呤在人体内的最终代谢产物, 人体每天产生的尿酸三分之一是经由胃肠道排泄, 三分之二经由肾脏排泄, 其中经由肾脏排泄的尿酸有90%会在阴离子转运蛋白的作用下重吸收[5], 在这一重吸收过程中起主要作用的是分布于肾近曲小管上皮细胞上的尿酸转运体1 (uric acid transporter 1, URAT1)[6].研究表明, 高于90%的高尿酸血症是由于尿酸排泄减少造成的, 其余大约10%是由于尿酸产生过多造成的, 因此, 通过抑制URAT1的重吸收来促进尿酸的排泄, 是一种非常有前景的治疗痛风及高尿酸血症的治疗方法[7, 8]. Lesinurad (RDEA594) 是由美国Ardea Biosciences研发的新型URAT1抑制剂(图式1), 于2015年年底经美国食品与药品监督管理局(FDA)批准在美国上市, 随后在2016年初在欧盟被批准上市[9, 10].
本实验室前期在研究URAT1抑制剂的过程中, 系统研究了一种基于lesinurad的苗头化合物的构效关系, 最终发现了候选药物1, 1是一种含有柔性的萘三氮唑甲烷结构的强效URAT1抑制剂候选药物, 其对URAT1的体外抑制活性是其母体lesinurad的76倍(Scheme 1)[11]. 1的分子结构中在萘环和三唑环之间含一个能够使整个分子相对母体lesinurad更加具有柔性的CH2连接臂(linker), 这个CH2连接臂是候选药物1的活性显著提高的重要因素.为了进一步研究该CH2连接臂上的取代基的体积对活性的影响, 我们设计了7个新颖的含有大位阻结构的化合物, 在该CH2连接臂上引入了7种体积大小不同的取代基(Scheme 2).研究结果发现, CH2连接臂上引入取代基后活性普遍显著降低, 且引入取代基后导致的分子柔性越差, 活性越弱.本研究的结果获得的构效关系对URAT1抑制剂的结构设计具有重要的指导作用.
1 结果与讨论
1.1 目标化合物合成
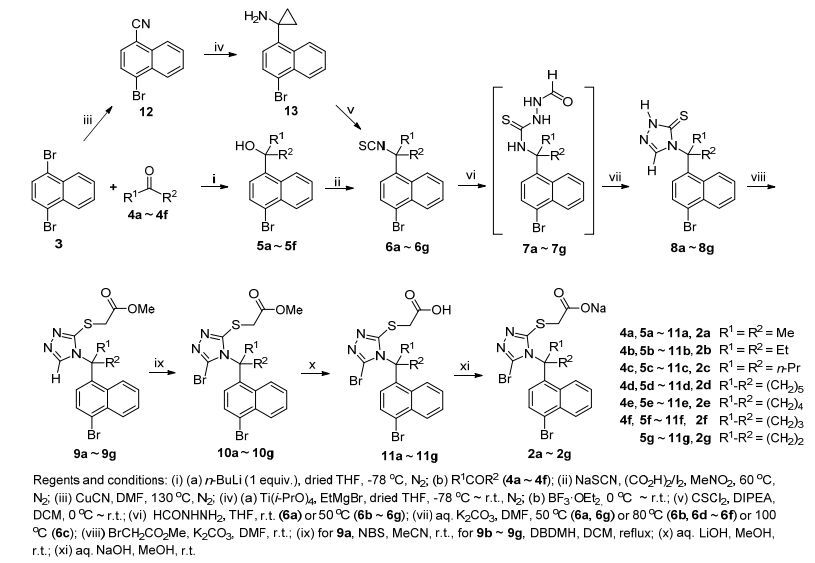
目标化合物2a~2g的合成路线见Scheme 2.起始原料1, 4-二溴萘(3)在干燥的四氢呋喃(THF)中-78 ℃下与1 equiv.的正丁基锂反应, 分子中两个Br原子中的一个发生Br-Li交换, 生成4-溴-1-萘基锂活性中间体, 后者与后加入的酮4a~4f发生亲核加成反应, 得到叔醇5a~5f.在(CO2H)2/I2存在下叔醇5a~5f与NaSCN在硝基甲烷中60 ℃下反应得到异硫氰酸酯6a~6f[12, 13].需要特别注意的是, 此反应为SN1反应, 反应物的加入顺序尤为重要:将(CO2H)2/I2加入到叔醇5a~5f和NaSCN的硝基甲烷溶液中后, (CO2H)2/I2先与5a~5f反应, 使后者转变为叔碳正离子, 而后碳正离子迅速与体系中已经存在的SCN-负离子反应, 得到产物6a~6f; 如果在叔醇5a~5f的硝基甲烷溶液中先加入(CO2H)2/I2再加入NaSCN, 则5a~5f生成的叔碳正离子由于来不及与后加入的SCN-反应, 而会发生自身消除形成烯类的副产物, 最终几乎得不到期望化合物.在氮气气氛中1, 4-二溴萘(3)与CuCN在N, N-二甲基甲酰胺(DMF)溶液中130 ℃下反应得到4-溴-1-萘甲腈(12)[14].萘甲腈12与Ti(i-PrO)4和EtMgBr先在干燥的THF中-78 ℃至室温下反应, 而后0 ℃下与加入的BF3•OEt2反应得到环丙基胺13 (Kulinkovich-Szymoniak Cyclopropanation)[15~17].胺13与硫光气在二异丙基乙基胺(DIPEA)存在下在CH2Cl2中冰水浴冷却下反应, 得到异硫氰酸酯6g.异硫氰酸酯6a~6g与甲酰肼在THF中反应, 得到的中间体7a~7g在DMF中加热下(50~100 ℃)经K2CO3催化关环, 得到1, 2, 4-三唑-3-硫酮8a~8g.在甲酰肼对异硫氰酸酯6a~6g的亲核加成反应中, 因为取代基R1和R2的体积不同, 所以反应温度差异也较大, 对于体积较小的偕二甲基(6a), 室温下即可顺利反应; 而体积较大的其他基团(6b~6g), 由于会对异硫氰酸酯造成较大的位阻, 则需要在较高温度下(50 ℃)才能顺利进行.由于同样的原因, 在中间体7a~7g的关环反应中, 也观察到了类似的位阻现象:对于偕二甲基(7a)和环丙基(7g), 在50 ℃即可顺利关环, 对于偕二乙基(7b), 环己基(7d), 环戊基(7e)和环丁基(7f), 需要80 ℃方可关环, 而对于偕二正丙基(7c), 则需要100 ℃才能关环.硫酮8a~8g在K2CO3存在下在DMF中室温下与溴乙酸甲酯反应, 被顺利S-烷基化, 得到2-硫代乙酸甲酯9a~9g.三氮唑9a~9g与溴化剂反应, 在5-位被溴化, 得到溴代三氮唑10a~10g.由于底物9a~9g中取代基R1和R2体积不同, 取代基带来的位阻对溴化反应的影响也不一样:当取代基为偕二甲基(9a)时, 底物9a可被N-溴代丁二酰亚胺(NBS)在乙腈中室温下顺利溴化; 但是当取代基为其他较大的基团(9b~9g)时, 底物则需要使用具有更强溴化能力的1, 3-二溴-5, 5-二甲基海因(DBDMH)在回流的CH2Cl2中才能溴化[18].需要特别指出的是, 上述溴化反应的产率都普遍较低(20%~65%), 可能与溴化反应的条件比较剧烈造成的副反应比较多有关.乙酸酯10a~10g在MeOH中室温下被LiOH水解得到对应的酸11a~11g.为了增加在生物活性测试中的溶解性, 酸11a~11g在MeOH中用NaOH转化为对应的钠盐2a~2g.
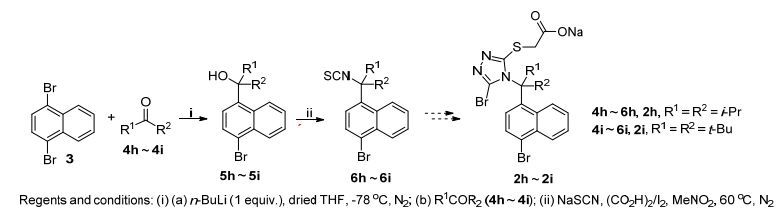
为了在2a~2c的基础上进一步增加取代基R1和R2的体积, 来更加系统地探索该类取代基对活性的影响, 我们也设计了具有更大取代基的2h和2i两个化合物(Scheme 3).如Scheme 3所示, 使用前述与制备叔醇3a~3f一样的方法, 由1, 4-二溴萘(3)可顺利制得叔醇5h和5i; 但是在下一步使用前述由5a~5f制备6a~6f一样的方法来处理5h和5i时, 发现没有反应发生, 可能是由于偕二异丙基和偕二叔丁基的体积太大, (CO2H)2难以与叔醇5h和5i反应使之产生叔碳正离子.
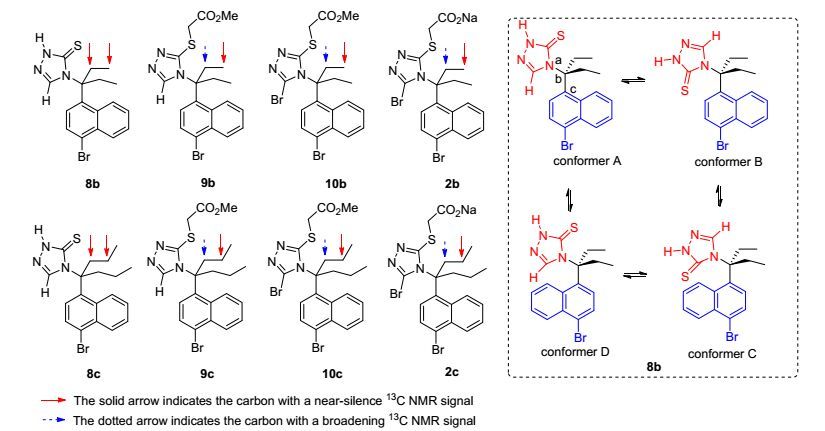
在对所有中间体和最终产物的NMR数据研究的过程中, 我们发现: Scheme 2所示的反应路线中自三唑硫酮开始往后的所有偕二乙基(8b~10b, 2b)和偕二正丙基(8c~10c, 2c)取代的中间体和最终产物的1H NMR图谱中的偕二乙基和偕二正丙基上的信号都显著变宽; 在13C NMR图谱中, 上述化合物呈现出如图 1所示的规律, 即, 在上述所有分子中, 无论偕二乙基还是偕二正丙基, 与季碳相连的沿碳链方向的两个碳原子均出现了“几乎不出峰(near-silence)”或者“宽峰(broadening)”的情况[19, 20], 而偕二正丙基分子中正丙基末端甲基则出现一个尖锐单峰.针对这一“异常”情况, 经过仔细研究后我们发现, 季碳上链接的四个基团的位阻非常大, 所以上述分子沿着“a-b”轴和“b-c”轴出现了旋转障碍, 即, 在核磁共振测试的温度条件下, 上述分子沿着上述两个轴不能自由旋转, 产生了类似旋转异构体的四个构象A, B, C和D (图 1, 以分子8b作为代表来说明), 这四种不同构象之间相互转化的位阻造成了其1H NMR信号变宽[19]和13C NMR信号几乎不出峰[20].需要说明的是, 偕二正丙基中远端的甲基在碳谱上出现了一个尖锐的单峰, 可能是上述不同构象的的转化对其影响较小(因为该远端甲基距离两个轴很远).
1.2 体外活性测试
化合物2a~2g, 1和对照阳性药lesinurad对人URAT1的体外抑制活性测试结果见表 1.由表 1数据可见, 总体上来讲, 在候选药物1的萘环和三氮唑之间的CH2连接臂上引入取代基后, 对URAT1的抑制活性普遍明显下降, 仅有2d和2e两个化合物的活性与lesinurad类似.当取代基R1和R2为直链的烷基(2a~2c)时, 烷基的链越长, 活性越差; 当它们为环烷基(2d~2g)时, 基本上是环越小活性越差.进一步研究上述规律, 可以发现, 在CH2连接臂上引入取代基后, 因为旋转位阻而造成分子柔性普遍降低, 柔性越差的分子, 活性越差:在直链的烷基系列(2a~2c)中, 烷基链越长, 体积越大, 则旋转位阻越大, 整个分子的柔性越低; 而在环烷基系列(2d~2g)中, 环越小, 环的刚性越强, 则整个分子的柔性越低.上述结果说明, 候选药物1中的萘环和三唑环之间的CH2连接臂不能容忍任何取代基, 因为任何取代基都会导致分子的柔性下降.
 表 1
化合物2a~2g, 1和lesinurad对人URAT1体外抑制活性的实验结果
Table 1.
Results for in vitro assays of compounds 2a~2g, 1 and lesinurad against human URAT1
表 1
化合物2a~2g, 1和lesinurad对人URAT1体外抑制活性的实验结果
Table 1.
Results for in vitro assays of compounds 2a~2g, 1 and lesinurad against human URAT1

Compound R1 R2 IC50a/(μmol•L-1) Lesinurad — — 7.18±0.65 1 H H 0.094±0.012b 2a Me Me 24.7±3.40 2b Et Et 35.41±4.72 2c n-Pr n-Pr >50 2d (CH2)5 9.16±1.32 2e (CH2)4 7.38±0.89 2f (CH2)3 26.63±3.63 2g (CH2)2 39.50±4.90 — — — aExperiments were performed in triplicate and values were expressed as mean±SD. aReported value[14] measured under the identical conditions. 2 结论
为了进一步研究URAT1抑制剂候选药物1的构效关系, 我们对其萘环和三唑环之间的CH2连接臂进行了取代基修饰, 活性测试结果发现所合成的7个含有大位阻结构的化合物2a~2g活性相比1普遍降低, 且引入取代基后的分子的柔性越差, 活性越不好, 显示了候选药物1中萘环和三唑环之间的CH2连接臂上不能容忍其他取代基.本研究获得的构效关系对URAT1抑制剂的结构设计具有重要的指导作用.我们同时研究了大体积的取代基造成的分子旋转障碍对NMR信号峰型的影响.
3 实验部分
3.1 仪器与试剂
RY-2显微熔点测定仪(天津天光光学仪器有限公司), 温度未校正; Bruker AV400型核磁共振仪, DMSO-d6为溶剂, TMS为内标; Agilent Q-TOF 6510型高分辨质谱仪, 电喷雾离子化技术(ESI), 直接进样法进样.无水溶剂采用标准方法制备.起始原料1, 4-二溴萘(3)和酮4a~4f, 4h, 4i为市售.
3.2 化合物合成
3.2.1 叔醇5a~5f和5h~5i的合成
1, 4-二溴萘(3, 57.19 g, 200 mmol)溶于干燥的四氢呋喃(600 mL)溶液中, 搅拌, 在氮气气氛中降温至-78 ℃, 用注射器向体系中缓慢滴加1.6 mol/L的n-BuLi的正己烷溶液(125 mL, 200 mmol).滴加完毕后, 在此温度下继续搅拌0.5 h, 而后用注射器向体系中滴加4a~4f (240 mmol).滴加完毕后, 在该温度下再反应0.5 h, 然后在室温下继续搅拌1 h.将反应混合物小心倒入冰水中, 用CH2Cl2 (600 mL×3) 萃取, 合并有机相, 用饱和食盐水(500 mL)洗涤, 无水硫酸钠干燥, 在旋转蒸发仪上蒸去溶剂, 得到的残余物经柱层析[V(EtOAc)/V(n-hexane)=1/15]分离即可得到目标化合物5a~5f.
2-(4-溴-1-萘基)-2-丙醇(5a):无色油状液体, 37.12 g, 收率70%. 1H NMR (DMSO-d6, 400 MHz) δ: 8.97 (d, J=8.0 Hz, 1H), 8.17~8.20 (m, 1H), 7.80 (d, J=8.0 Hz, 1H), 7.55~7.66 (m, 2H), 7.48 (d, J=8.0 Hz, 1H), 5.38 (s, 1H), 1.69 (s, 6H).
3-(4-溴-1-萘基)-3-戊醇(5b):无色油状液体, 44.57 g, 收率76%. 1H NMR (DMSO-d6, 400 MHz) δ: 8.73 (d, J=8.4 Hz, 1H), 8.18~8.20 (m, 1H), 7.82 (d, J=8.0 Hz, 1H), 7.54~7.64 (m, 3H), 4.94 (s, 1H), 2.11~2.20 (m, 2H), 1.93~2.02 (m, 2H), 0.62 (t, J=7.4 Hz, 6H).
4-(4-溴-1-萘基)-4-庚醇(5c):白色固体, 56.54 g, 收率88%. m.p. 104~105 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 8.69 (d, J=7.6 Hz, 1H), 8.17~8.20 (m, 1H), 7.80 (d, J=8.0 Hz, 1H), 7.55~7.64 (m, 2H), 4.98 (s, 1H), 2.08~2.16 (m, 2H), 1.88~1.96 (m, 2H), 1.17~1.26 (m, 2H), 0.84~0.89 (m, 2H), 0.72 (t, J=7.4 Hz, 6H).
1-(4-溴-1-萘基)-1-环己醇(5d):白色固体, 50.66 g, 收率83%. m.p. 75~76 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 9.09 (d, J=8.4 Hz, 1H), 8.18 (dd, J=1.2, 8.4 Hz, 1H), 7.80 (d, J=8.0 Hz, 1H), 7.55~7.64 (m, 2H), 7.46 (d, J=8.0 Hz, 1H), 5.09 (s, 2H), 2.10~2.13 (m, 2H), 1.80~1.95 (m, 4H), 1.66~1.70 (m, 1H), 1.53~1.56 (m, 2H), 1.23~1.32 (m, 1H).
1-(4-溴-1-萘基)-1-环戊醇(5e):白色固体, 44.26 g, 收率76%. m.p. 67.5~68.5 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 8.74 (d, J=8.4 Hz, 1H), 8.16~8.18 (m, 1H), 7.78 (d, J=8.0 Hz, 1H), 7.57~7.66 (m, 2H), 7.49 (d, J=8.0 Hz, 1H), 5.14 (s, 1H), 2.06~2.20 (m, 4H), 1.85~1.98 (m, 2H), 1.70~1.75 (m, 2H).
1-(4-溴-1-萘基)-1-环丁醇(5f):白色固体, 42.68 g, 收率77%. m.p. 100~101 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 8.37 (d, J=8.4 Hz, 1H), 8.17 (d, J=8.4 Hz, 1H), 7.82 (d, J=7.6 Hz, 1H), 7.57~7.67 (m, 2H), 7.43 (d, J=8.0 Hz, 1H), 5.73 (s, 1H), 2.61~2.67 (m, 2H), 2.44~2.51 (m, 2H), 1.94~2.04 (m, 1H), 1.48~1.58 (m, 1H).
3-(4-溴-1-萘基)-2, 4-二甲基-3-戊醇(5h):白色固体, 38.55 g, 收率60%. m.p. 64~65 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 9.75 (s, 1H), 8.15 (d, J=7.2 Hz, 1H), 7.80 (d, J=7.6 Hz, 1H), 7.55~7.57 (m, 1H), 7.48 (s, 1H), 7.27 (s, 1H), 4.73 (brs, 1H), 2.44 (s, 2H), 0.83 (s, 6H), 0.74 (s, 6H).
3-(4-溴-1-萘基)-2, 2, 4, 4-四甲基-3-戊醇(5i):白色固体, 34.93 g, 收率50%. m.p. 114~115 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 8.57~8.59 (m, 1H), 8.16~8.18 (m, 1H), 8.12 (d, J=8.8 Hz, 1H), 7.85 (d, J=8.8 Hz, 1H), 7.56~7.60 (m, 2H), 4.72 (s, 1H), 1.13 (s, 18H).
3.2.2 4-溴-1-萘腈(12)的合成
向干燥的圆底烧瓶中加入1, 4-二溴萘(3, 57.19 g, 200 mmol), CuCN (10.75 g, 120 mmol)和DMF (600 mL), 搅拌, 在氮气保护下升温至130 ℃, 并在该温度下反应12 h.反应结束后冷却至室温, 将体系倒入CH2Cl2(1000 mL)中, 搅拌3 h, 所得浆状物通过硅藻土抽滤, 滤液用饱和食盐水(500 mL×5) 洗涤, 无水硫酸钠干燥, 在旋转蒸发仪上蒸去溶剂, 残余物经柱层析[V(EtOAc)/V(n-hexane)=1/30]纯化得化合物12.白色固体, 11.60 g, 收率25%. m.p. 97~98 ℃(文献值[14, 21]103~104 ℃); 1H NMR (DMSO-d6, 400 MHz) δ: 8.28~8.31 (m, 1H, Ar-H), 8.15~8.17 (m, 1H, Ar-H), 8.07 (s, 2H, Ar-H), 7.86~7.92 (m, 2H, Ar-H).此数据与文献[14, 21]报道基本一致.
3.2.3 1-(4-溴-1-萘基)-1-环丙胺(13)的合成
向干燥的三口烧瓶中加入12 (10.44 g, 45 mmol)和干燥的THF (100 mL), 搅拌, 氮气置换三次后用注射器慢慢滴加Ti(i-PrO)4 (14.07 g, 49.50 mmol).将体系降温至-78 ℃, 非常缓慢地滴加1.0 mol/L的EtMgBr的THF溶液(99 mL, 99 mmol), 滴加完毕后在此温度下继续搅拌30 min, 撤去低温浴, 体系缓慢升至室温(约1 h).然后将体系置于冰水浴中冷却, 用注射器缓慢滴加BF3•Et2O(12.77 g, 90 mmol), 滴加完毕后撤去冰水浴, 继续反应1 h.向体系中依次滴加1 mol/L的盐酸溶液和1 mol/L的氢氧化钠溶液淬灭反应, 而后倒入冰水中, 用CH2Cl2 (200 mL×3) 萃取, 合并有机相后用饱和食盐水(200 mL)洗涤一次, 无水硫酸钠干燥, 在旋转蒸发仪上蒸去溶剂, 所得物经柱层析[V(EtOAc)/V(n-hexane)=1/2]纯化得化合物13.黄色液体, 2.36 g, 收率20%. 1H NMR (DMSO-d6, 400 MHz) δ: 8.54~8.57 (m, 1H), 8.15~8.18 (m, 1H), 7.78 (d, J=7.6 Hz, 1H), 7.66~7.71 (m, 2H), 7.40 (d, J=8.0 Hz, 1H), 2.46 (brs, 2H), 1.03~1.06 (m, 2H), 0.85~0.88 (m, 2H).
3.2.4 异硫氰酸酯6a~6g的合成
由5a~5f制备6a~6f:向圆底烧瓶中加入5a~5f (100 mmol)和MeNO2 (15 mL), 然后依次加入NaSCN (24.32 g, 300 mmol), (CO2H)2 (9 g, 100 mmol)和I2 (12.69 g, 50 mmol).在氮气保护下升温至60 ℃, 薄层色谱(TLC)检测直到反应完成(5a, 约12 h; 5b~5f, 约1 h).反应结束后, 将反应混合物降至室温, 倒入溶有Na2S2O3 (5 g)的冰水中, 充分搅拌后通过硅藻土抽滤, 收集的滤液用二氯甲烷(300 mL×3) 萃取, 合并有机相, 用饱和Na2CO3溶液(300 mL×5) 洗涤, 无水硫酸钠干燥, 在旋转蒸发仪上蒸去溶剂, 残余物经柱层析(n-hexane)纯化得化合物6a~6f.
由13制备6g:向圆底烧瓶中加入反应物13 (2.23 g, 8.5 mmol)和CH2Cl2(20 mL), 搅拌, 置于冰水浴中冷却, 加入DIPEA (3.30 g, 25.5 mmol).向体系中缓慢的滴加CSCl2 (1.08 g, 9.35 mmol), 滴加完毕后撤去冰浴, 恢复至室温, 并在室温下反应1 h, TLC显示反应完成.反应混合物倒入冰水(100 mL)中, 用1 mol/L盐酸溶液调节pH值为5~6, 用CH2Cl2 (100 mL×3) 萃取, 合并有机相, 用饱和食盐水洗涤有机相, 无水硫酸钠干燥, 抽滤收集滤液, 在旋转蒸发仪上蒸去溶剂, 残余物经柱层析[V(EtOAc)/V(n-hexane)=1/2]纯化得化合物6g.
(2-(4-溴-1-萘基)-2-正丙基)异硫氰酸酯(6a):白色晶体, 24.50 g, 收率80%. m.p. 96~96.5 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 8.51~8.53 (m, 1H), 8.28~8.30 (m, 1H), 7.90 (d, J=8.0 Hz, 1H), 7.73~7.79 (m, 2H), 7.54 (d, J=8.0 Hz, 1H), 2.02 (s, 6H).
(3-(4-溴-1-萘基)-3-正戊基)异硫氰酸酯(6b):无色油状液体, 18.05 g, 收率54%. 1H NMR (DMSO-d6, 400 MHz) δ: 8.41~8.43 (m, 1H), 8.27~8.29 (m, 1H), 7.93 (d, J=8.0 Hz, 1H), 7.67~7.75 (m, 2H), 7.51 (d, J=8.4 Hz, 1H), 2.49~2.58 (m, 2H), 2.28~2.37 (m, 2H), 0.78 (t, J=7.4 Hz, 6H).
(4-(4-溴-1-萘基)-4-正庚基)异硫氰酸酯(6c):白色固体, 32.60 g, 收率90%. m.p. 92~93 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 8.41~8.43 (m, 1H), 8.26~8.28 (m, 1H), 7.91 (d, J=8.0 Hz, 1H), 7.68~7.74 (m, 2H), 7.51 (d, J=8.0 Hz, 1H), 2.45~2.53 (m, 2H), 2.21~2.29 (m, 2H), 1.20~1.31 (m, 2H), 0.99~1.08 (m, 2H), 0.80 (t, J=7.4 Hz, 6H).
(1-(4-溴-1-萘基)-1-环己基)异硫氰酸酯(6d):白色固体, 10.35 g, 收率30%. m.p. 87~88 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 8.49~8.53 (m, 1H), 8.26~8.29 (m, 1H), 7.89 (d, J=8.4 Hz, 1H), 7.70~7.76 (m, 2H), 7.53 (d, J=8.0 Hz, 1H), 2.49~2.54 (m, 2H), 1.99~2.06 (m, 2H), 1.71~1.81 (m, 5H), 1.33~1.40 (m, 1H).
(1-(4-溴-1-萘基)-1-环戊基)异硫氰酸酯(6e):白色固体, 13.29 g, 收率40%. m.p. 88~89 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 8.43~8.45 (m, 1H), 8.25~8.28 (m, 1H), 7.87 (d, J=8.0 Hz, 1H), 7.72~7.77 (m, 2H), 7.56 (d, J=8.0 Hz, 1H), 2.68~2.72 (m, 2H), 2.27~2.34 (m, 2H), 1.88~1.96 (m, 4H).
(1-(4-溴-1-萘基)-1-环丁基)异硫氰酸酯(6f):无色油状液体, 26.09 g, 收率82%. 1H NMR (DMSO-d6, 400 MHz) δ: 8.25~8.27 (m, 1H), 8.02~8.06 (m, 1H), 7.91 (d, J=8.0 Hz, 1H), 7.72~7.77 (m, 2H), 7.53 (d, J=7.6 Hz, 1H), 2.90~2.94 (m, 4H), 2.24~2.35 (m, 1H), 1.82~1.91 (m, 1H).
(1-(4-溴-1-萘基)-1-环丙基)异硫氰酸酯(6g):无色油状液体, 2.33 g, 收率90%. 1H NMR (DMSO-d6, 400 MHz) δ: 8.43~8.46 (m, 1H), 8.24~8.26 (m, 1H), 7.89 (d, J=7.6 Hz, 1H), 7.76~7.85 (m, 2H), 7.59 (d, J=7.6 Hz, 1H), 1.72~1.76 (m, 2H), 1.39~1.42 (m, 2H).
3.2.5 4-取代-2, 3-二氢-4H-1, 2, 4-三唑啉-3-硫酮8a~8g的合成
向圆底烧瓶中加入6a~6g (7 mmol)和THF (20 mL), 搅拌, 再加入甲酰肼(14 mmol), 于室温(6a)或者50 ℃ (6b~6g)下反应过夜, TLC显示反应完成.在旋转蒸发仪上蒸去溶剂, 残余物即为7a~7g, 不经进一步处理直接用于下一步反应.上述7a~7g粗品溶于DMF (30 mL)中, 加入由K2CO3 (0.97 g, 7 mmol)和H2O (6 mL)配制的溶液, 在氮气保护下升温反应(7a和7g, 50 ℃; 7b和7d~7f, 80 ℃; 7c, 100 ℃), 直到TLC检测反应完成(一般24~36 h).反应混合物降至室温, 倒入冰水中, 用1 mol/L稀盐酸调节pH值为6, 逐渐有白色固体析出.抽滤收集固体, 用EtOAc重结晶得到8a~8g的纯品.
4-(1-(4-溴-1-萘基)-1-甲基乙基)-2, 3-二氢-4H-1, 2, 4-三唑啉-3-硫酮(8a):白色固体, 1.61 g, 收率66%. m.p. 202~204 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 13.50 (brs, 1H), 8.81 (s, 1H), 8.21 (d, J=8.0 Hz, 1H), 7.88 (d, J=7.6 Hz, 1H), 7.59~7.61 (m, 2H), 7.45 (s, 2H), 2.16 (s, 6H); 13C NMR (DMSO-d6, 100 MHz) δ: 165.34, 140.55, 138.75, 131.66, 131.11, 129.25, 127.89, 126.78, 126.69, 126.03, 123.85, 122.14, 62.01, 28.75; HRMS calcd for C15H1479BrN3SNa[M+Na]+ 369.9984, found 369.9984; calcd for C15H1481BrN3SNa [M+Na]+ 371.9964, found 371.9947.
4-(1-(4-溴-1-萘基)-1-乙基丙基)-2, 3-二氢-4H-1, 2, 4-三唑啉-3-硫酮(8b).白色固体, 1.58 g, 收率60%. m.p. 218~220 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 13.50 (brs, 1H), 8.89 (brs, 1H), 8.21 (d, J=8.4 Hz, 1H), 7.87 (d, J=8.0 Hz, 1H), 7.53~7.59 (m, 2H), 7.38~7.45 (m, 2H), 3.24 (brs, 1H), 2.66 (brs, 1H), 2.49 (brs, 2H), 0.57 (brs, 6H); 13C NMR (DMSO-d6, 100 MHz) δ: 165.39, 141.48, 136.42, 131.66, 131.40, 128.79, 127.96, 127.85, 126.65, 126.60, 123.42, 122.17, 67.99, 7.74; HRMS calcd for C17H1879BrN3SNa[M+Na]+ 398.0297, found 398.0297; calcd for C17H1881BrN3SNa [M+Na]+ 400.0277, found 400.0279.
4-(1-(4-溴-1-萘基)-1-正丙基丁基)-2, 3-二氢-4H-1, 2, 4-三唑啉-3-硫酮(8c):白色固体, 2.12 g, 收率75%. m.p. 195~197 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 13.46 (brs, 1H), 8.87 (brs, 1H), 8.21 (d, J=8.8 Hz, 1H), 7.86 (d, J=8.0 Hz, 1H), 7.54~7.59 (m, 2H), 7.38~7.46 (m, 2H), 2.43 (brs, 2H), 0.47~1.17 (m, 12H); 13C NMR (DMSO-d6, 100 MHz) δ: 165.36, 141.33, 136.92, 131.66, 131.40, 128.84, 127.96, 127.58, 126.65, 126.62, 123.42, 122.13, 67.36, 16.92, 13.94; HRMS calcd for C19H2279Br-N3SNa [M+Na]+ 426.0610, found 426.0613; calcd for C19H2281BrN3SNa [M+Na]+ 428.0590, found 428.0593.
4-(1-(4-溴-1-萘基)-1-环己基)-2, 3-二氢-4H-1, 2, 4-三唑啉-3-硫酮(8d):白色固体, 1.71 g, 收率63%. m.p. 190~192 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 13.47 (brs, 1H), 9.00 (s, 1H), 8.22 (d, J=8.0 Hz, 1H), 7.90 (d, J=8.0 Hz, 1H), 7.73~7.76 (m, 2H), 7.57~7.64 (m, 1H), 7.41~7.47 (m, 1H), 3.09~3.13 (m, 2H), 2.66~2.70 (m, 2H), 1.50~1.56 (m, 6H); 13C NMR (DMSO-d6, 100 MHz) δ: 165.34, 141.90, 136.30, 131.97, 131.21, 129.15, 128.69, 128.03, 126.55, 126.49, 124.28, 122.50, 64.98, 34.59, 24.82, 22.31; HRMS calcd for C18H1879BrN3SNa[M+Na]+ 410.0297, found 410.0291; calcd for C18H1881BrN3-SNa [M+Na]+ 412.0277, found 412.0270.
4-(1-(4-溴-1-萘基)-1-环戊基)-2, 3-二氢-4H-1, 2, 4-三唑啉-3-硫酮(8e):白色固体, 2.49 g, 收率95%. m.p. 185 ℃ (dec.); 1H NMR (DMSO-d6, 400 MHz) δ: 13.48 (brs, 1H), 8.86 (s, 1H), 8.21 (d, J=8.0 Hz, 1H), 7.87 (d, J=8.0 Hz, 1H), 7.79 (d, J=8.8 Hz, 1H), 7.71 (d, J=8.0 Hz, 1H), 7.61 (t, J=7.6 Hz, 1H), 7.47~7.51 (m, 1H), 3.26 (brs, 2H), 2.43~2.46 (m, 2H), 1.72~1.82 (m, 4H); 13C NMR (DMSO-d6, 100 MHz) δ: 165.40, 141.57, 136.45, 131.71, 131.50, 128.79, 127.75, 127.70, 126.78, 126.76, 124.53, 122.34, 72.38, 36.74, 22.02; HRMS calcd for C17H1679BrN3SNa [M+Na]+ 396.0141, found 396.0137; calcd for C17H1681BrN3SNa [M+Na]+ 398.0120, found 398.0122.
4-(1-(4-溴-1-萘基)-1-环丁基)-2, 3-二氢-4H-1, 2, 4-三唑啉-3-硫酮(8f):白色固体, 1.92 g, 收率76%. m.p. 193~195 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 13.50 (brs, 1H), 9.20 (s, 1H), 8.20~8.22 (m, 1H), 7.99 (d, J=8.0 Hz, 1H), 7.93 (t, J=8.2 Hz, 2H), 7.64 (t, J=7.4 Hz, 1H), 7.55~7.58 (m, 1H), 3.42~3.49 (m, 2H), 2.99~3.06 (m, 2H), 2.01~2.07 (m, 1H), 1.81~1.88 (m, 1H); 13C NMR (DMSO-d6, 100 MHz) δ: 165.32, 141.17, 134.52, 131.60, 130.77, 129.56, 128.42, 127.83, 127.05, 127.02, 124.43, 122.60, 65.29, 33.14, 15.76; HRMS calcd for C16H1479BrN3SNa [M+Na]+ 381.9984, found 381.9979; calcd for C16H1481BrN3SNa [M+Na]+ 383.9964, found 383.9963.
4-(1-(4-溴-1-萘基)-1-环丙基)-2, 3-二氢-4H-1, 2, 4-三唑啉-3-硫酮(8g):白色固体, 2.18 g, 收率90%. m.p. 240 ℃ (dec.); 1H NMR (DMSO-d6, 400 MHz) δ: 13.51 (brs, 1H), 9.13 (s, 1H), 8.81~8.83 (m, 1H), 8.25 (d, J=7.6 Hz, 1H), 8.18~8.20 (m, 1H), 7.90 (d, J=8.0 Hz, 1H), 7.68~7.75 (m, 2H), 1.81~1.84 (m, 2H), 1.54~1.57 (m, 2H); 13C NMR (DMSO-d6, 100 MHz) δ: 166.96, 142.66, 132.97, 132.51, 132.11, 131.13, 128.92, 127.61, 127.47, 127.38, 124.84, 123.08, 37.56, 14.23; HRMS calcd for C15H1379BrN3S [M+H]+ 346.0008, found 346.0002; calcd for C15H1381BrN3S [M+H]+ 347.9988, found 347.9982.
3.2.6 2-((4-取代-4H-1, 2, 4-三唑-3-基)硫)乙酸甲酯(9a~9g)的合成
向圆底烧瓶中加入8a~8g (4 mmol), K2CO3 (1.11 g, 8 mmol)和DMF (20 mL), 搅拌, 室温下滴加溴乙酸甲酯(0.73 g, 4.80 mol), 滴加完毕后, 反应混合物在氮气保护下室温搅拌, 直到TLC显示反应完成(一般12 h).反应混合物倒入冰水(100 mL)中, 用CH2Cl2 (150 mL×3) 萃取, 合并有机相, 并用饱和食盐水洗涤, 无水硫酸钠干燥, 抽滤收集滤液, 在旋转蒸发仪上蒸去溶剂, 残余物经柱层析[V(EtOAc)/V(n-hexane)=1/0~1/1]纯化得化合物9a~9g.
2-((4-(1-(4-溴-1-萘基)-1-甲基乙基)-4H-1, 2, 4-三唑-3-基)硫)乙酸甲酯(9a):白色固体, 1.09 g, 收率65%. m.p. 151~152 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 9.06 (s, 1H), 8.24~8.26 (m, 1H), 7.97 (d, J=8.0 Hz, 1H), 7.74 (d, J=8.4 Hz, 1H), 7.60~7.64 (m, 1H), 7.41~7.45 (m, 1H), 7.33 (d, J=8.8 Hz, 1H), 3.76 (s, 2H), 3.48 (s, 3H), 2.11 (s, 6H); 13C NMR (DMSO-d6, 100 MHz) δ: 168.44, 148.20, 143.26, 137.46, 131.91, 131.30, 129.45, 128.02, 127.39, 127.23, 126.34, 123.92, 123.84, 61.01, 52.14, 33.87, 29.52; HRMS calcd for C18H1979BrN3O2S[M+H]+ 420.0376, found 420.0374; calcd for C18H19-81BrN3O2S [M+H]+ 422.0355, found 422.0349.
2-((4-(1-(4-溴-1-萘基)-1-乙基丙基)-4H-1, 2, 4-三唑-3-基)硫)乙酸甲酯(9b):白色固体, 1.43 g, 收率80%. m.p. 150~152 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 9.10 (s, 1H), 8.25 (d, J=8.0 Hz, 1H), 7.97 (d, J=8.4 Hz, 1H), 7.70 (d, J=8.0 Hz, 1H), 7.61 (t, J=7.6 Hz, 1H), 7.37~7.41 (m, 1H), 7.31 (d, J=8.4 Hz, 1H), 3.76 (brs, 2H), 3.46 (s, 3H), 2.53~2.63 (m, 4H), 0.56 (brs, 6H); 13C NMR (DMSO-d6, 100 MHz) δ: 168.35, 148.37, 144.25, 135.54, 131.90, 131.57, 129.07, 128.08, 127.30, 127.17, 123.95, 123.44, 66.95, 52.10, 33.80, 26.90, 7.86; HRMS calcd for C20H2379BrN3O2S [M+H]+ 448.0689, found 448.0682; calcd for C20H2381BrN3O2S [M+H]+ 450.0668, found 450.0669.
2-((4-(1-(4-溴-1-萘基)-1-正丙基丁基)-4H-1, 2, 4-三唑-3-基)硫)乙酸甲酯(9c):白色固体, 1.64 g, 收率86%. m.p. 161~163 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 9.09 (s, 1H), 8.24 (d, J=8.4 Hz, 1H), 7.96 (d, J=8.0 Hz, 1H), 7.71 (d, J=8.0 Hz, 1H), 7.61 (t, J=7.6 Hz, 1H), 7.39 (t, J=7.6 Hz, 1H), 7.32 (d, J=8.8 Hz, 1H), 3.76 (brs, 2H), 3.46 (s, 3H), 2.54~2.60 (m, 4H), 1.27 (brs, 2H), 0.82 (brs, 8H); 13C NMR (DMSO-d6, 100 MHz) δ: 168.35, 148.31, 144.06, 135.96, 131.89, 131.54, 129.08, 128.05, 127.82, 127.28, 127.15, 123.90, 123.41, 66.28, 52.05, 36.97, 33.70, 13.78; HRMS calcd for C22H2779BrN3O2SNa[M+Na]+ 498.0821, found 498.0812; calcd for C22H27-81BrN3O2SNa [M+Na]+ 500.0801, found 500.0792.
2-((4-(1-(4-溴-1-萘基)-1-环己基)-4H-1, 2, 4-三唑-3-基)硫)乙酸甲酯(9d):白色泡沫, 1.44 g, 收率78%; 1H NMR (DMSO-d6, 400 MHz) δ: 9.25 (s, 1H), 8.24 (d, J=8.4 Hz, 1H), 7.98 (d, J=8.4 Hz, 1H), 7.87 (d, J=8.0 Hz, 1H), 7.61 (t, J=7.8 Hz, 1H), 7.55 (d, J=8.8 Hz, 1H), 7.42 (t, J=7.4 Hz, 1H), 3.79 (s, 2H), 3.47 (s, 3H), 2.76~2.80 (m, 2H), 2.58~2.62 (m, 2H), 1.54~1.62 (m, 6H); 13C NMR (DMSO-d6, 100 MHz) δ: 168.42, 148.25, 144.02, 135.40, 132.18, 131.48, 128.98, 128.92, 128.10, 127.11, 124.12, 123.98, 63.80, 52.10, 36.13, 33.96, 24.65, 22.42; HRMS calcd for C21H2279BrN3O2SNa[M+Na]+ 482.0508, found 482.0510; calcd for C21H2281BrN3O2S[M+Na]+ 484.0488, found 484.0488.
2-((4-(1-(4-溴-1-萘基)-1-环戊基)-4H-1, 2, 4-三唑-3-基)硫)乙酸甲酯(9e):白色固体, 1.25 g, 收率70%. m.p. 152~153 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 9.11 (s, 1H), 8.23 (d, J=8.0 Hz, 1H), 7.95 (d, J=8.0 Hz, 1H), 7.82 (d, J=8.0 Hz, 1H), 7.61~7.68 (m, 2H), 7.45~7.49 (m, 1H), 3.81 (s, 2H), 3.48 (s, 3H), 2.94 (brs, 2H), 2.49 (brs, 2H), 1.85~1.87 (m, 2H), 1.73 (brs, 2H); 13C NMR (DMSO-d6, 100 MHz) δ: 168.46, 148.35, 144.48, 136.02, 131.89, 131.81, 129.05, 127.82, 127.32, 126.87, 124.41, 123.78, 71.20, 52.13, 37.85, 33.91, 22.18; HRMS calcd for C20H2079BrN3O2SNa[M+Na]+ 468.0352, found 468.0344; calcd for C20H2081BrN3O2SNa[M+Na]+ 470.0331, found 470.0335.
2-((4-(1-(4-溴-1-萘基)-1-环丁基)-4H-1, 2, 4-三唑-3-基)硫)乙酸甲酯(9f):白色泡沫, 1.26 g, 收率73%. 1H NMR (DMSO-d6, 400 MHz) δ: 9.48 (s, 1H), 8.22 (d, J=8.4 Hz, 1H), 7.99 (d, J=8.0 Hz, 1H), 7.89 (d, J=8.0 Hz, 1H), 7.78 (d, J=8.4 Hz, 1H), 7.66 (t, J=7.6 Hz, 1H), 7.55 (t, J=7.6 Hz, 1H), 3.88 (s, 2H), 3.53 (s, 3H), 3.04~3.16 (m, 4H), 2.09~2.15 (m, 1H), 1.88~1.94 (m, 1H); 13C NMR (DMSO-d6, 100 MHz) δ: 168.50, 148.23, 144.43, 134.89, 131.86, 130.74, 128.77, 127.85, 127.65, 127.53, 127.45, 124.26, 123.63, 64.30, 52.21, 34.05, 33.86, 15.55; HRMS calcd for C19H1979BrN3O2S [M+H]+ 432.0376, found 432.0376; calcd for C19H1981BrN3O2S [M+H]+ 434.0355 (Br), found 434.0357.
2-((4-(1-(4-溴-1-萘基)-1-环丙基)-4H-1, 2, 4-三唑-3-基)硫)乙酸甲酯(9g):白色固体, 1.12 g, 收率67%. m.p. 155~156 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 9.33 (s, 1H), 8.70~8.72 (m, 1H), 8.20~8.22 (m, 1H), 7.95 (d, J=8.0 Hz, 1H), 7.89 (d, J=8.0 Hz, 1H), 7.71~7.78 (m, 2H), 4.07 (s, 2H), 3.58 (s, 3H), 1.81~1.84 (m, 2H), 1.64~1.67 (m, 2H); 13C NMR (DMSO-d6, 100 MHz) δ: 168.55, 150.18, 145.82, 132.97, 132.24, 131.39, 130.02, 129.14, 127.92, 127.51, 124.56, 123.61, 52.33, 37.27, 33.76, 14.09; HRMS calcd for C18H1779BrN3O2S[M+H]+ 418.0219, found 418.0214; calcd for C18H1781BrN3O2S[M+H]+ 420.0199 (Br), found 420.0202.
3.2.7 2-((5-溴-4-取代-4H-1, 2, 4-三氮唑-3-基)硫)乙酸甲酯(10a~10g)的合成
由9a制备10a:向圆底烧瓶中加入反应物9a (0.84 g, 2 mmol)和MeCN (15 mL), 加入NBS (0.41 g, 2.3 mmol), 在室温下搅拌过夜, TLC检测反应完成.
由9b~9g制备10b~10g:向圆底烧瓶中加入反应物9b~9g (2 mmol)和CH2Cl2(15 mL), 于室温下加入DBDMH (0.57 g, 2 mmol).然后回流过夜, TLC检测反应完成.
反应结束后, 将体系降温(如有需要)倒入冰水中, 用CH2Cl2 (50 mL×3) 萃取, 合并有机相, 用饱和碳酸钾溶液洗涤(100 mL×5), 最后用饱和食盐水洗涤, 无水硫酸钠干燥, 抽滤收集滤液, 在旋转蒸发仪上蒸去溶剂, 残余物经柱层析纯化[V(EtOAc)/V(n-hexane)=1/8~1/15]得化合物10a~10g.
2-((5-溴-4-(1-(4-溴-1-萘基)-1-甲基乙基)-4H-1, 2, 4-三唑-3-基)硫)乙酸甲酯(10a):泡沫, 0.32 g, 收率32%. 1H NMR (DMSO-d6, 400 MHz) δ: 8.25 (d, J=8.0 Hz, 1H), 7.97 (d, J=8.0 Hz, 1H), 7.73 (d, J=8.4 Hz, 1H), 7.64 (t, J=7.6 Hz, 1H), 7.44~7.48 (m, 1H), 7.14 (d, J=8.8 Hz, 1H), 4.08 (s, 2H), 3.68 (s, 3H), 2.09 (s, 6H); 13C NMR (DMSO-d6, 100 MHz) δ: 169.11, 158.37, 138.23, 131.80, 131.03, 129.47, 128.31, 127.98, 127.53, 127.27, 125.76, 123.36, 123.30, 65.66, 52.35, 33.16, 29.38; HRMS calcd for C18H18Br2N3O2S [M+H]+ 499.9461, found 499.9468.
2-((5-溴-4-(1-(4-溴-1-萘基)-1-乙基丙基)-4H-1, 2, 4-三唑-3-基)硫)乙酸甲酯(10b):泡沫, 0.24 g, 收率23%. 1H NMR (DMSO-d6, 400 MHz) δ: 8.25 (d, J=8.4 Hz, 1H), 7.96 (d, J=8.0 Hz, 1H), 7.60~7.67 (m, 2H), 7.42 (t, J=7.8 Hz, 1H), 7.13 (d, J=8.8 Hz, 1H), 4.08 (s, 2H), 3.67 (s, 3H), 2.61 (brs, 4H), 0.58 (brs, 6H); 13C NMR (DMSO-d6, 100 MHz) δ: 169.07, 158.14, 136.44, 131.83, 131.31, 129.06, 128.81, 128.04, 127.41, 127.17, 123.34, 123.05, 71.45, 52.29, 33.18, 27.47, 7.66; HRMS calcd for C20H21Br2N3O2SNa[M+Na]+ 549.9593, found 549.9599.
2-((5-溴-4-(1-(4-溴-1-萘基)-1-正丙基丁基)-4H-1, 2, 4-三唑-3-基)硫)乙酸甲酯(10c):白色固体, 0.22 g, 收率20%. m.p. 134~135 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 8.24 (d, J=8.4 Hz, 1H), 7.95 (d, J=8.0 Hz, 1H), 7.68 (d, J=8.0 Hz, 1H), 7.62 (t, J=7.6 Hz, 1H), 7.40~7.44 (m, 1H), 7.12 (d, J=8.8 Hz, 1H), 4.08 (s, 2H), 3.67 (s, 3H), 2.57 (brs, 4H), 1.39 (brs, 1H), 1.17 (brs, 1H), 0.81 (brs, 8H); 13C NMR (DMSO-d6, 100 MHz) δ: 169.10, 158.15, 136.88, 131.81, 131.26, 129.10, 128.72, 128.04, 127.43, 127.19, 127.16, 123.31, 123.03, 70.72, 52.29, 37.66, 33.22, 13.95; HRMS calcd for C22H25Br2N3O2SNa[M+Na]+ 577.9906, found 577.9903.
2-((5-溴-4-(1-(4-溴-1-萘基)-1-环己基)-4H-1, 2, 4-三唑-3-基)硫)乙酸甲酯(10d):泡沫, 0.38 g, 收率35%. 1H NMR (DMSO-d6, 400 MHz) δ: 8.22 (d, J=8.4 Hz, 1H), 7.95 (d, J=8.0 Hz, 1H), 7.80 (d, J=8.0 Hz, 1H), 7.61 (t, J=7.4 Hz, 1H), 7.39~7.46 (m, 2H), 4.12 (s, 2H), 3.69 (s, 3H), 2.87 (brs, 2H), 2.34 (brs, 2H), 1.64~1.78 (m, 4H), 1.46 (brs, 2H); 13C NMR (DMSO-d6, 100 MHz) δ: 169.09, 158.26, 138.57, 131.95, 131.25, 129.19, 128.90, 127.96, 127.34, 127.15, 126.61, 123.57, 123.19, 67.77, 52.32, 36.50, 33.07, 24.66, 21.81; HRMS calcd for C21H21Br2-N3O2SNa[M+Na]+ 561.9593, found 561.9595.
2-((5-溴-4-(1-(4-溴-1-萘基)-1-环戊基)-4H-1, 2, 4-三唑-3-基)硫)乙酸甲酯(10e):泡沫, 0.40 g, 收率38%. 1H NMR (DMSO-d6, 400 MHz) δ: 8.22 (d, J=8.4 Hz, 1H), 7.93 (d, J=8.0 H, 1Hz), 7.78 (d, J=8.0 Hz, 1H), 7.63 (t, J=7.4 Hz, 1H), 7.55 (d, J=8.4 Hz, 1H), 7.48 (t, J=7.6 Hz, 1H), 4.09 (s, 2H), 3.70 (s, 3H), 2.98 (brs, 2H), 2.49 (brs, 2H), 1.74~1.82 (m, 4H); 13C NMR (DMSO-d6, 100 MHz) δ: 169.08, 157.98, 136.55, 131.81, 131.67, 129.04, 128.52, 127.67, 127.40, 127.25, 126.32, 124.16, 123.16, 75.72, 52.29, 38.49, 33.06, 22.55; HRMS calcd for C20H19Br2N3O2SNa [M+Na]+ 547.9436, found 547.9439.
2-((5-溴-4-(1-(4-溴-1-萘基)-1-环丁基)-4H-1, 2, 4-三唑-3-基)硫)乙酸甲酯(10f):白色固体, 0.66 g, 收率65%. m.p. 133~135 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 8.21 (d, J=8.4 Hz, 1H), 7.97 (d, J=8.0 Hz, 1H), 7.91 (d, J=8.0 Hz, 2H), 7.66 (t, J=7.6 Hz, 1H), 7.54~7.58 (m, 1H), 4.08 (s, 2H), 3.70 (s, 3H), 3.28 (brs, 2H), 3.06 (s, 2H), 2.04~2.09 (m, 1H), 1.86~1.91 (m, 1H); 13C NMR (DMSO-d6, 100 MHz) δ: 169.10, 158.00, 134.85, 131.83, 130.98, 128.73, 128.14, 127.74, 127.62, 127.50, 127.38, 124.75, 123.24, 67.18, 52.32, 33.70, 33.09, 15.35; HRMS calcd for C19H18Br2N3O2S [M+H]+ 511.9461, found 511.9451.
2-((5-溴-4-(1-(4-溴-1-萘基)-1-环丙基)-4H-1, 2, 4-三唑-3-基)硫)乙酸甲酯(10g):白色固体, 0.60 g, 收率60%. m.p. 129~131 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 8.98~9.02 (m, 1H), 8.19~8.23 (m, 1H), 8.07 (d, J=8.0 Hz, 1H), 7.94 (d, J=8.0 Hz, 1H), 7.72~7.76 (m, 2H), 4.21 (s, 2H), 3.61 (s, 3H), 2.00~2.03 (m, 2H), 1.83~1.86 (m, 2H); 13C NMR (DMSO-d6, 100 MHz) δ: 168.36, 152.88, 133.05, 132.12, 131.63, 131.30, 130.55, 129.04, 127.85, 127.49, 127.35, 125.31, 123.92, 52.46, 39.07, 34.33, 15.34; HRMS calcd for C18H16Br2N3O2S[M+H]+ 497.9304, found 497.9311.
3.2.8 2-((5-溴-4-取代-4H-1, 2, 4-三唑-3-基)硫)乙酸11a~11g的合成
向圆底烧瓶中加入10a~10g (0.4 mmol)和MeOH (10 mL), 于室温下加入由LiOH•H2O (0.34 g, 0.8 mmol)和水(1 mL)配制的溶液, 而后室温下搅拌, TLC检测直到反应结束(一般5 h内).反应混合物倒入冰水(50 mL)中, 滴加浓盐酸调节pH为1~2.用CH2Cl2萃取(25mL×3), 合并有机相, 无水硫酸钠干燥, 抽滤收集滤液, 在旋转蒸发仪上蒸去溶剂, 残余物经短柱柱层析[11b, V(EtOAc)/V(MeOH)=1/2]或者打浆[11a和11c~11g, V(EtOAc)/V(n-hexane)=1/1]得化合物11a~11g的纯品.
2-((5-溴-4-(1-(4-溴-1-萘基)-1-甲基乙基)-4H-1, 2, 4-三唑-3-基)硫)乙酸(11a):白色固体, 0.29 g, 收率75%. m.p. 177~178 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 12.82 (brs, 1H), 8.24 (d, J=8.4 Hz, 1H), 7.96 (d, J=8.0 Hz, 1H), 7.73 (d, J=8.4 Hz, 1H), 7.63 (t, J=7.6 Hz, 1H), 7.43~7.48 (m, 1H), 7.17 (d, J=8.8 Hz, 1H), 3.98 (s, 2H), 2.10 (s, 6H); HRMS calcd for C17H16Br2N3O2S [M+H]+ 485.9304, found 485.9304.
2-((5-溴-4-(1-(4-溴-1-萘基)-1-乙基丙基)-4H-1, 2, 4-三唑-3-基)硫)乙酸(11b):白色泡沫, 0.17 g, 收率82%. 1H NMR (DMSO-d6, 400 MHz) δ: 12.80 (brs, 1H), 8.24 (d, J=8.4 Hz, 1H), 7.96 (d, J=8.0 Hz, 1H), 7.60~7.67 (m, 2H), 7.42 (t, J=7.8 Hz, 1H), 7.16 (d, J=8.8 Hz, 1H), 3.98 (s, 2H), 2.62 (brs, 4H), 0.58 (brs, 6H); HRMS calcd for C19H19Br2N3O2SNa [M+Na]+535.9436, found 535.9434.
2-((5-溴-4-(1-(4-溴-1-萘基)-1-正丙基丁基)-4H-1, 2, 4-三唑-3-基)硫)乙酸(11c).白色固体, 0.17 g, 收率78%. m.p. 146~147 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 12.80 (brs, 1H), 8.24 (d, J=8.0 Hz, 1H), 7.94 (d, J=8.0 Hz, 1H), 7.67 (d, J=8.0 Hz, 1H), 7.61 (t, J=7.6 Hz, 1H), 7.40~7.44 (m, 1H), 7.16 (d, J=8.8 Hz, 1H), 3.97 (s, 2H), 2.58 (brs, 4H), 1.41 (brs, 1H), 1.16 (brs, 1H), 0.81~0.85 (m, 8H); HRMS calcd for C21H23Br2N3O2SNa[M+Na]+ 563.9749, found 563.9740.
2-((5-溴-4-(1-(4-溴-1-萘基)-1-环己基)-4H-1, 2, 4-三唑-3-基)硫)乙酸(11d):白色固体, 0.16 g, 收率75%. m.p. 157~159 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 12.86 (brs, 1H), 8.22 (d, J=8.0 Hz, 1H), 7.95 (d, J=8.0 Hz, 1H), 7.80 (d, J=8.4 Hz, 1H), 7.58~7.62 (m, 1H), 7.44~7.45 (m, 2H), 4.01 (s, 2H), 2.88 (brs, 2H), 2.35 (brs, 2H), 1.65~1.78 (m, 4H), 1.46 (brs, 2H); HRMS calcd for C20H19Br2N3O2SNa[M+Na]+ 547.9436, found 547.9429.
2-((5-溴-4-(1-(4-溴-1-萘基)-1-环戊基)-4H-1, 2, 4-三唑-3-基)硫)乙酸(11e):白色固体, 0.14 g, 收率70%. m.p. 120~121 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 12.82 (brs, 1H), 8.21 (d, J=8.4 Hz, 1H), 7.92 (d, J=8.0 Hz, 1H), 7.78 (d, J=8.0 Hz, 1H), 7.58~7.65 (m, 2H), 7.49 (t, J=7.6 Hz, 1H), 3.99 (s, 2H), 2.99 (brs, 2H), 2.49 (brs, 2H), 1.80 (brs, 4H); HRMS calcd for C19H17Br2N3-O2SNa [M+Na]+ 533.9280, found 533.9277.
2-((5-溴-4-(1-(4-溴-1-萘基)-1-环丁基)-4H-1, 2, 4-三唑-3-基)硫)乙酸(11f):白色固体, 0.15 g, 收率73%. m.p. 161~162 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 12.83 (brs, 1H), 8.21 (d, J=8.4 Hz, 1H), 7.97 (d, J=8.0 Hz, 2H), 7.91 (d, J=8.0 Hz, 1H), 7.65 (t, J=7.4 H, 1Hz), 7.56~7.60 (m, 1H), 3.99 (s, 2H), 3.30 (brs, 2H), 3.05 (brs, 2H), 2.04~2.10 (m, 1H), 1.84~1.91 (m, 1H); HRMS calcd for C18H16Br2N3O2S[M+H]+ 497.9304, found 497.9307.
2-((5-溴-4-(1-(4-溴-1-萘基)-1-环丙基)-4H-1, 2, 4-三唑-3-基)硫)乙酸(11g):白色固体, 0.14 g, 收率75%. m.p. 207~208 ℃; 1H NMR (DMSO-d6, 400 MHz) δ: 12.93 (brs, 1H), 8.99~9.01 (m, 1H), 8.21~8.23 (m, 1H), 8.08 (d, J=8.0 Hz, 1H), 7.94 (d, J=8.0 Hz, 1H), 7.72~7.76 (m, 2H), 4.13 (s, 2H), 2.00~2.04 (m, 2H), 1.83~1.86 (m, 2H); HRMS calcd for C17H14Br2N3O2S[M+H]+ 483.9148, found 483.9144.
3.2.9 2-((5-溴-4-取代-4H-1, 2, 4-三唑-3-基)硫)乙酸钠2a~2g的合成
向圆底烧瓶中加入11a~11g (0.28 mmol, 准确称量精确到小数点后4位)和MeOH (5 mL), 于室温下逐滴加入NaOH (0.1120 g, 0.28 mmol)和水(0.5 mL)的水溶液, 反应0.5 h.然后在旋转蒸发仪上蒸去溶剂, 残余物用CH2Cl2溶解后再蒸去溶剂(5 mL×5), 所得残余物在真空油泵上干燥得2a~2g的纯品.
2-((5-溴-4-(1-(4-溴-1-萘基)-1-甲基乙基)-4H-1, 2, 4-三唑-3-基)硫)乙酸钠(2a):白色固体, 0.14 g, 收率100%. m.p. 103 ℃ (dec.); 1H NMR (DMSO-d6, 400 MHz) δ: 8.23 (d, J=8.4 Hz, 1H), 7.95 (d, J=8.4 Hz, 1H), 7.72 (d, J=8.0 Hz, 1H), 7.62 (t, J=7.6 Hz, 1H), 7.45~7.49 (m, 1H), 7.22 (d, J=8.8 Hz, 1H), 3.80 (s, 2H), 2.10 (s, 6H); 13C NMR (DMSO-d6, 100 MHz) δ: 170.01, 160.31, 138.49, 131.78, 131.12, 129.43, 127.91, 127.60, 127.20, 125.65, 123.50, 123.23, 65.35, 37.28, 29.47; HRMS calcd for C17H14Br2N3O2S [M-Na]- 483.9158, found 483.9160.
2-((5-溴-4-(1-(4-溴-1-萘基)-1-乙基丙基)-4H-1, 2, 4-三唑-3-基)硫)乙酸钠(2b):白色固体, 0.15 g, 收率100%. m.p. 165 ℃ (dec.); 1H NMR (DMSO-d6, 400 MHz) δ: 8.24 (d, J=8.4 Hz, 1H), 7.95 (d, J=8.0 Hz, 1H), 7.58~7.65 (m, 2H), 7.43 (t, J=7.4 Hz, 1H), 7.22 (d, J=8.8 Hz, 1H), 3.69 (s, 2H), 2.61~2.62 (m, 4H), 0.58 (br s, 6H); 13C NMR (DMSO-d6, 100 MHz) δ: 169.78, 160.94, 136.77, 131.79, 131.42, 129.04, 127.97, 127.88, 127.40, 127.33, 127.12, 123.20, 123.17, 70.99, 39.23, 27.58; HRMS calcd for C19H18Br2N3O2S[M-Na]- 511.9471, found 511.9472.
2-((5-溴-4-(1-(4-溴-1-萘基)-1-正丙基丁基)-4H-1, 2, 4-三唑-3-基)硫)乙酸钠(2c):白色固体, 0.16 g, 收率100%. m.p. 155 ℃ (dec.); 1H NMR (DMSO-d6, 400 MHz) δ: 8.23 (d, J=8.4 Hz, 1H), 7.94 (d, J=8.0 Hz, 1H), 7.66 (d, J=8.4 Hz, 1H), 7.60 (t, J=7.6 Hz, 1H), 7.41~7.45 (m, 1H), 7.22 (d, J=8.8 Hz, 1H), 3.71 (s, 2H), 2.58 (brs, 4H), 1.41 (brs, 1H), 1.17 (brs, 1H), 0.81 (brs, 8H); 13C NMR (DMSO-d6, 100 MHz) δ: 170.05, 160.70, 137.15, 131.78, 131.36, 129.06, 127.96, 127.81, 127.40, 127.09, 123.19, 123.1 70.32, 38.78, 37.66, 13.93; HRMS calcd for C21H22Br2N3O2S[M-Na]- 539.9784, found 539.9779.
2-((5-溴-4-(1-(4-溴-1-萘基)-1-环己基)-4H-1, 2, 4-三唑-3-基)硫)乙酸钠(2d):白色固体, 0.15 g, 收率100%. m.p. 116 ℃ (dec.); 1H NMR (DMSO-d6, 400 MHz) δ: 8.21 (d, J=8.4 Hz, 1H), 7.94 (d, J=8.0 Hz, 1H), 7.80 (d, J=8.0 Hz, 1H), 7.59 (t, J=7.6 Hz, 1H), 7.51 (d, J=8.4 Hz, 1H), 7.44 (t, J=7.6 Hz, 1H), 3.79 (s, 2H), 2.88 (brs, 2H), 2.36 (brs, 2H), 1.77~1.80 (m, 2H), 1.65 (brs, 4H), 1.46 (brs, 2H); 13C NMR (DMSO-d6, 100 MHz) δ: 169.65, 160.37, 138.65, 131.92, 131.36, 129.12, 128.01, 127.85, 127.35, 127.07, 126.64, 123.72, 123.09, 67.43, 37.80, 36.57, 24.68, 21.97; HRMS calcd for C20H18Br2N3O2S[M-Na]- 523.9471, found 523.9471.
2-((5-溴-4-(1-(4-溴-1-萘基)-1-环戊基)-4H-1, 2, 4-三唑-3-基)硫)乙酸钠(2e):白色固体, 0.15 g, 收率100%. m.p. 120 ℃ (dec.); 1H NMR (DMSO-d6, 400 MHz) δ: 8.21 (d, J=8.4 Hz, 1H), 7.91~7.93 (m, 1H), 7.76 (d, J=8.0 Hz, 1H), 7.60~7.67 (m, 2H), 7.50 (t, J=7.6 Hz, 1H), 3.69 (s, 2H), 2.99 (brs, 2H), 2.49 (brs, 2H), 1.79 (brs, 4H); 13C NMR (DMSO-d6, 100 MHz) δ: 169.75, 160.71, 136.86, 131.81, 131.74, 129.05, 127.64, 127.40, 127.23, 126.30, 124.30, 123.04, 75.23, 38.99, 38.57, 22.68; HR-MS calcd for C19H16Br2N3O2S[M-Na]- 509.9315, found 509.9309.
2-((5-溴-4-(1-(4-溴-1-萘基)-1-环丁基)-4H-1, 2, 4-三唑-3-基)硫)乙酸钠(2f):白色固体, 0.15 g, 收率100%. m.p. 134 ℃ (dec.); 1H NMR (DMSO-d6, 400 MHz) δ: 8.19 (d, J=8.0 Hz, 1H), 8.04 (d, J=8.8 Hz, 1H), 7.96 (d, J=8.0 Hz, 1H), 7.90 (d, J=8.0 Hz, 1H), 7.63 (t, J=7.6 Hz, 1H), 7.56 (, J=7.6 Hz t, 1H), 3.78 (s, 2H), 3.30 (brs, 2H), 3.04 (brs, 2H), 2.03~2.08 (m, 1H), 1.82~1.88 (m, 1H); 13C NMR (DMSO-d6, 100 MHz) δ: 169.78, 159.95, 135.06, 131.82, 131.07, 128.69, 127.60, 127.52, 127.47, 127.39, 124.99, 123.15, 67.79, 37.31, 33.74, 15.38; HRMS calcd for C18H14Br2N3O2S[M-Na]- 495.9158, found 495.9159.
2-((5-溴-4-(1-(4-溴-1-萘基)-1-环丙基)-4H-1, 2, 4-三唑-3-基)硫)乙酸钠(2g):白色固体, 0.14 g, 收率100%. m.p. 172 ℃ (de.c); 1H NMR (DMSO-d6, 400 MHz) δ: 9.04~9.07 (m, 1H), 8.18~8.21 (m, 1H), 8.08 (d, J=8.0 Hz, 1H), 7.93 (d, J=8.0 Hz, 1H), 7.69~7.74 (m, 2H), 3.93 (s, 2H), 2.04 (s, 2H), 1.80~1.83 (m, 2H); 13C NMR (DMSO-d6, 100 MHz) δ: 169.15, 154.42, 133.38, 132.19, 131.66, 131.28, 130.00, 129.06, 127.80, 127.43, 127.30, 125.44, 123.77, 38.95, 38.50, 15.45; HRMS calcd for C17H12Br2N3O2S[M-Na]- 481.9002, found 481.9004.
3.3 体外活性测试
使用待测化合物2a~2g抑制稳定表达了URAT1的HEK293细胞对[8-14C]尿酸的摄取的方法来评价待测化合物对URAT1的体外抑制强度, 利用lesinurad作为阳性对照[11].待测化合物对URAT1的抑制强度以半抑制浓度(half maximal inhibitory concentration, IC50)来表示.
辅助材料(Supporting Information) 所有最终产物和在本文讨论中涉及到的部分中间体的1H NMR和13C NMR图谱.这些材料可以免费从本刊网站(http://sioc-journal.cn/)上下载.
-
-
[1]
Richette, P.; Bardin, T. Lancet 2010, 375, 318. doi: 10.1016/S0140-6736(09)60883-7
-
[2]
Punzi, L.; Scanu, A.; Ramonda, R.; Oliviero, F. Autoimmun. Rev. 2012, 12, 66. doi: 10.1016/j.autrev.2012.07.024
-
[3]
Dalbeth, N.; Merriman, T. R.; Stamp, L. K. Lacent 2016, 388, 2039.
-
[4]
Kuo, C, F.; Grainge, M. J.; Zhang, W. Y.; Doherty, M. Nat. Rev. Rheumatol. 2015, 11, 649. doi: 10.1038/nrrheum.2015.91
-
[5]
Hyndman, D.; Liu, S.; Miner, J. N. Curr. Rheumatol. Rep. 2016, 18, 34. doi: 10.1007/s11926-016-0587-7
-
[6]
Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Cha, S. H.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T.; Matsuo, H.; Kikuchi, Y.; Oda, T.; Ichida, K.; Hosoya, T.; Shimokata, K.; Niwa, T.; Kanai, Y.; Endou, H. Nature 2002, 417, 447. doi: 10.1038/nature742
-
[7]
Cai, W.; Liu, W.; Liu, C.; Wang, J.; Zhao, G. Chin. J. Struct. Chem. 2017, 36, 897.
-
[8]
田禾, 吴景卫, 刘钰强, 谢亚非, 王建武, 赵桂龙, 有机化学, 2017, 37, 1748. http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract345979.shtmlTian, H.; Wu, J.; Liu, Y.; Xie, Y.; Wang, J.; Zhao, G. Chin. J. Org. Chem. 2017, 37, 1748(in Chinese). http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract345979.shtml
-
[9]
Hoy, S. M. Drugs 2016, 76, 509. doi: 10.1007/s40265-016-0550-y
-
[10]
Shen, Z.; Yeh, L. T.; Wallach, K.; Zhu, N.; Kerr, B.; Gillen, M. Clin. Drug Invest. 2016, 36, 443. doi: 10.1007/s40261-016-0386-y
-
[11]
Zhang, X.; Wu, J.; Liu, W.; Liu, Y.; Xie, Y.; Shang, Q.; Zhou, Z.; Xu, W.; Tang, L.; Wang, J.; Zhao, G. Med. Chem. 2017, 13, 260. doi: 10.2174/1573406412666160915163002
-
[12]
Miyake, H.; Nakao, Y.; Sasaki, M. Chem. Lett. 2006, 11, 1262.
-
[13]
Miyake, H.; Nakao, Y.; Sasaki, M. Tetrahedron 2007, 63, 10433. doi: 10.1016/j.tet.2007.08.014
-
[14]
张宪生, 刘钰强, 谢亚非, 李川, 辛晓, 徐为人, 汤立达, 赵桂龙, 现代药物与临床, 2015, 30, 1179. http://www.cnki.com.cn/Article/CJFDTOTAL-SYQY201603027.htmZhang, X.; Liu, Y.; Xie, Y.; Li, C.; Xin, X.; Xu, W.; Tang, L.; Zhao, G. Drugs Clin. 2015, 30, 1179(in Chinese). http://www.cnki.com.cn/Article/CJFDTOTAL-SYQY201603027.htm
-
[15]
Bertus, P.; Szymoniak, J. J. Org. Chem. 2003, 68, 7133. doi: 10.1021/jo034710+
-
[16]
Michailidis, F. R.; Pupier, M.; Besnard, C.; Burgi, T.; Alexakis, A. Org. Lett. 2014, 16, 4988. doi: 10.1021/ol5022355
-
[17]
Li, W. J.; Gao, J, J.; Lorenz, J. C.; Xu, J. H.; Johnson, J.; Ma, S. L.; Lee, H.; Grinberg, N.; Busacca, C. A.; Lu, B.; Senanayake, C. H. Org. Process Res. Dev. 2012, 16, 836. doi: 10.1021/op300059b
-
[18]
Haudrechy, A.; Chassaing, C.; Riche, C.; Langlois, Y. Tetrahedron 2000, 56, 3181. doi: 10.1016/S0040-4020(00)00227-1
-
[19]
Murayama, K.; Yoshioka, T. Tetrahedron Lett. 1968, 11, 1363.
-
[20]
Glaser, R.; Hillebrand, R.; Wycoff, W.; Camasta, C.; Gates, K, S. J. Org. Chem. 2015, 80, 4360. doi: 10.1021/acs.joc.5b00080
-
[21]
Cakmak, O.; Kahveci, I.; Demirta, I.; Hokelek, T.; Smith, K. Collect. Czech. Chem. Commun. 2000, 65, 791.
-
[1]
-
表 1 化合物2a~2g, 1和lesinurad对人URAT1体外抑制活性的实验结果
Table 1. Results for in vitro assays of compounds 2a~2g, 1 and lesinurad against human URAT1

Compound R1 R2 IC50a/(μmol•L-1) Lesinurad — — 7.18±0.65 1 H H 0.094±0.012b 2a Me Me 24.7±3.40 2b Et Et 35.41±4.72 2c n-Pr n-Pr >50 2d (CH2)5 9.16±1.32 2e (CH2)4 7.38±0.89 2f (CH2)3 26.63±3.63 2g (CH2)2 39.50±4.90 — — — aExperiments were performed in triplicate and values were expressed as mean±SD. aReported value[14] measured under the identical conditions. -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 5
- 文章访问数: 1352
- HTML全文浏览量: 138


 下载:
下载:



 下载:
下载:

