 Figure Figure1.
Structures of β-amino esters and derivatives
Figure Figure1.
Structures of β-amino esters and derivatives

手性辅基诱导β-脱氢氨基酸酯的非均相不对称氢化
English
Pt/Al2O3 Catalyzed Diastereoselective Hydrogenation of β-Dehydro-amino Esters Induced by a Chiral Prosthetic Group
-
Key words:
- sitagliptin
- / diastereoselectivities
- / β-dehydroamino esters
- / Pt/Al2O3
- / heterogeneous hydrogenation
-
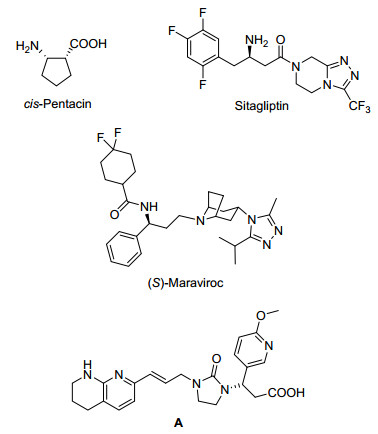
Enantiomerically pure β-amino acids and their derivatives are important and versatile synthetic building blocks in a variety of natural products and drugs, [1] such as sitagliptin, which is used for the treatment of diabetes, and some antiretroviral agents such as (S)-maraviroc and compound A (Figure 1).[2] Recently, more attention and popularity are gained due to their increasing significance for the synthesis of β-peptides, β-lactams and other biologically active compounds.[3] Consequently, it is of vital importance to develop new synthetic methods for the construction of β-amino acid and their derivatives. There have been numerous methods already available so far for the synthesis of chiral β-amino acids, such as modification of β-amino acid equivalent, [4] chiral Lewis acid catalyzed Mannich reaction, [5] asymmetric hydrogenation of β-dehydroamino ester derivatives[6] and so on.[7] Given its inherent atom economy and environment-friendly characteristics, it appears to be the most practical and economical way to focus on the area of asymmetric hydrogenation of β-dehydroamino ester derivatives.[8]
Transition-metal-catalyzed asymmetric hydrogenation of β-dehydroamino acids has attracted much attention in both industry and academy. Good to excellent enantioselectivities have been achieved by using ruthenium or rhodium with chiral phosphorus ligands (such as BINAP, Josiphos and Duanphos) as catalysts in homogeneous asymmetric hydrogenation of β-dehydroamino acid derivatives.[9] However, only a few applications have been applied to industrial production because of costly chiral ligands which are usually sensitive to the air and hard to synthesize.[10] Utilizing heterogeneous catalysts for practical productions is rather preferred reckoning their advantages in the easy separation and recycling of the catalyst, and the possibility of continuous operation.[11] They have been developed with modified catalyst, chiral prosthetic group and chiral auxiliary reagent involved.[12] For example, cinchona alkaloids modified palladium catalysts have been used for the asymmetric hydrogenation of α, β-unsaturated carboxylic acid, and good enantioselectivities were obtained for aryl-substituted substrates.[13] But this method could only be limited in α-dehydroamino substrates and hydrogenation of β-dehydroamino acid is still challenging.[14] Therefore a mild and effective asymmetric hydrogenation of β-dehydroamino acid is highly desirable. Herein we report chiral prosthetic group induced heterogeneous diastereoselective hydrogenation of β-dehydroa-mino acid with cheaper and easier prepared catalysts such as Pt/Al2O3compared with PtO2[15] for this transformation.
1 Results and discussion
We initiated our study by examining tert-butyl 3-(((1R)-1-phenylethyl)amino)-4-(2, 4, 5-trifluorophenyl)but-2-enoate (1a) which was synthesized from the according phenylacetic acid by several steps (Scheme 2) with Pt/C (5%) in i-PrOH at 35 ℃ under 2.02 MPa of H2for 22 h (Table 1, Entry 2).[16] It can be found that this reaction could occur with quantitative conversion but low diastereoselectivity. Then a series of common heterogeneous catalysts were tested. Unfortunately, these catalysts did little or no work on the hydrogenation except Pt/C (Table 1, Entries 1~3). Additives including Lewis acids and bases were examined whether they could control diastereoselectivities more efficiently by adjusting interactive force between substrates and catalysts.[17] However, none of them had a positive effect on conversion and diastereoselectivity simultaneously (Table 1, Entries 5~7). Similar negative effect was also observed when CH3COOH was added as additives (Table 1, Entry 4). At the same time, the influence of catalyst support on diastereoselectivity was also explored and it can be found that the diastereoselectivity and reactivity were sensitive to the catalyst model (Table 1, Entries 8~10). Only when Pt/Al2O3 (K0343) was used as catalyst the diastereoselectivity increased dramatically (Table 1, Entry 8).
Next, the effect of solvents on the conversion and diastereoselectivity was investigated. The results revealed that quantitative conversions in most solvents, but they had a large difference on the diastereoselectivities. Diastereoselectivity increased obviously along with enhancement of alcohols steric hindrance (Table 2, Entries 1~4). In addition, different solvents had a significant influence on diastereoselectivity. For example, nonprotic solvents were definitely harmful for diastereoselectivity in the hydrogenation of β-dehydroamino esters (Table 2, Entries 6~8). Moreover, there was even no conversion when the reaction was conducted in tetrahydrofuran (THF) (Table 2, Entry 8). Finally, we chose t-BuOH as the optimal solvent as it could give desired products with the highest drvalue (Table 2, Entry 4).
The hydrogen pressure also had a significant influence on diastereoselectivities. The dr value increased gradually when H2 pressure changed from 1.01 MPa to 8.10 MPa (Table 3, Entries 1~5), but slightly decreased when further raised to 10.1 MPa (Table 3, Entry 6). Higher temperature lowered the diastereoselectivity obviously as a result the diastereoselectivity dropped from 8.8:91.2 at 35 ℃ to 11.2:88.8 at 50 ℃ (Table 3, Entries 8 and 9). However, compared to the remarkable effect of increased temperature, there was little influence on dr value on the condition of the reduced temperature at 30 ℃ (Table 3, Entry 7). The best result was obtained at 35 ℃, which provided the corresponding product in full conversion and 7.5:92.5 dr value (Table 3, Entry 5). Therefore the optimal reaction conditions were set as follows: Pt/Al2O3(5%, K0343) as the catalyst in t-BuOH under 8.10 kPa of H2 at 35 ℃ for 22 h.
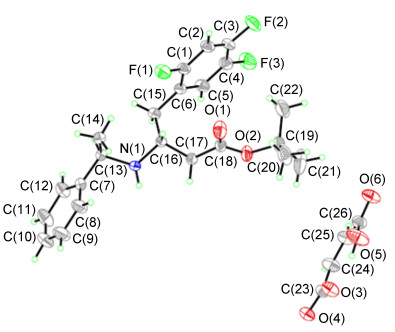
With the optimized reaction conditions in hand, a wide range of β-dehydroamino esters was subjected to the reaction, and the results were shown in Table 4. To our delight, a series of substrates were hydrogenated successfully, providing the corresponding β-amino esters with good to excellent yields and satisfying diastereoselectivities. The model substrate 1a was hydrogenated completely with 7.6:92.4 dr value (Table 4, Entry 1), and the corresponding hydrogenation product 2a was separated by adding maleic acid to form 4a which was further confirmed the absolute R configuration through X-ray diffraction analysis (Figure 2), giving the important intermediate of the (R)-Sitagliptin and its analogues. Surprisingly, when R2 was changed to methyl or isopropyl, the yields and selectivities declined significantly as the result with yields of 79% (dr: 14.4:85.6) and 76% (dr: 13.8:86.2), respectively (Table 4, Entries 2 and 3). In addition, substrates possessing different substitutes at ortho, meta and para positions of the phenyl moiety were successfully hydrogenated under the optimized conditions and a series of functional groups were tolerated (Table 4, Entries 4~10). For example, (Z)-tert-butyl 4-phenyl-3-((1-phenylethyl)-amino)but-2-enoate could be hydrogenated well with rather high diastereoselectivity (Table 4, Entry 4). It was noticed that substrates with electron-withdrawing group and electron-donating group at the para position of the phenyl moiety were hydrogenated in quantitative conversion with good diastereoselectivities (Table 4, Entries 5~7). Substrates with a chlorine atom substitute at the meta position of the phenyl ring gave the highest dr value which can be up to 5.2:94.8 (Table 4, Entry 8). However, the substrates owning ortho substitutes on the aromatic ring showed a completely different behavior considering both diastereoselectivity and reactivity. For substrate 1i, dr value was decreased obviously (Table 4, Entry 9). This result indicated that steric hindrance had a remarkable effect on diastereoselectivity. Furthermore, substrates containing o-methoxy substitutes on the phenyl ring could be transformed only with 70% yield and 23.8:76.2 dr value, which could be attributed to competing coordinating ability of o-OMe group with Pt catalyst (Table 4, Entry 10). Unfortunately, when the substrates was broadened to (Z)-tert-butyl 3-phenyl-3-((1-phenylethyl)amino)acrylate, low conversion ( < 10%) and dr value (38.4:61.6) were given even when the reaction time was prolonged to 30 h (Table 4, Entry 11).
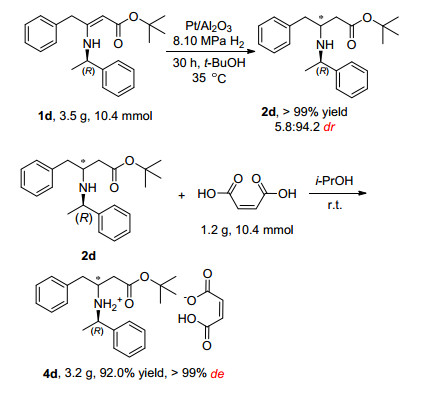
To highlight the practical utility of our method, the asymmetric hydrogenation was performed on a gram-scale (Scheme 1). Quantitative conversion was afforded when compound 1d (3.5 g, 10.4 mmol) was hydrogenated with 5% Pt/Al2O3 under 8.09 MPa of H2 at 35 ℃ in t-BuOH (50.0 mL) with 5.8:94.2 dr value which could be easily upgraded to 99% de by adding maleic acid (1 equiv.) to get pure product 4d. Recycled catalysts were reused for the hydrogenation of 1d with complete conversion for two times with 5.2:94.8 and 5.7:94.3 dr value, respectively.
2 Conclusions
In summary, we have successfully developed a highly efficient asymmetric hydrogenation of β-dehydroamino esters with a chiral benzyl as the easy-removing prosthetic group using Pt/Al2O3 (5%, K0343) catalytic system, and excellent yields and diastereoselectivities have been achieved with dr up to 5.2:94.8. Further studies are going on in our lab.
3 Experimental section
3.1 General considerations
Commercially available reagents and chemicals were used throughout without further purification other than those detailed below. i-PrOH was distilled over calcium hydride. MeOH and EtOH were distilled over magnesium under nitrogen. 1H NMR and 13C NMR spectra were performed on a Varian Plus-400 MHz. 1H NMR spectra were recorded at 400 MHz with TMS as internal standard. 13C NMR spectra were recorded at 100 MHz and referenced to the central peak of δ 77.00 for CDCl3. HRMS were performed under ESI ionization technique on a Q-TOF Premier mass spectrometer. HPLC was performed with X charge C18 column. Flash column chromatography was performed on silica gel (300~400 mesh).
3.2 Preparation of substrates
3.3 Gram-scale reaction
Compound 1d (3.5 g, 10.4 mmol) and Pt/Al2O3(5%, K0343, 175.0 mg) were dissolved in t-BuOH (50.0 mL), and the solution was hydrogenated under 80 atm H2 at 35oC for 30 h with 5.8: 94.2 dr value. To get pure single configuration product, maleic acid (1 equiv.) was added, so that concerning (3R)-tert-butyl 4-phenyl-3-(((1R)-1-pheny-lethyl)amino)butanoate (2d) salty form (3.2 g, 92.0% yield, > 99% de) could be separated from hydrogenation system.
Supporting Information 1H NMR, 13C NMR and 19F NMR spectra of substrates and products, and HPLC spectra of products. The Supporting Information is available free of charge via the Internet at http://sioc-journal.cn.
3.2.2 Preparation of tert-butyl (3R)-3-(((1R)-1-phen-ylethyl) amino)-4-(2, 4, 5-trifluorophenyl)butanoate (2a)
tert-Butyl 3-(((1R)-1-phenylethyl)amino)-4-(2, 4, 5-tri-fluorophenyl)but-2-enoate (1a) (78.0 mg, 0.2 mmol) and Pt/Al2O3(5%, K0343, 3.9 mg) were dissolved in tert-butanol (2.7 mL), and the solution was hydrogenated under 8.10 MPa H2 at 35 ℃ for 22 h. The crude product was purified by flash chromatography [V(methanol):V(water)=60:1].
(3R)-tert-Butyl 3-(((1R)-1-phenylethyl)amino)-4-(2, 4, 5-trifluorophenyl)butanoate (2a)[2]: Pale yellow liquid. Yield 95%, dr=7.6:92.4. [α]D25+24.8 (c 1.00, CHCl3) (R configuration hydrogenation product with maleic acid product 4a); 1H NMR (400 MHz, CDCl3) δ: 7.38~7.24 (m, 3H), 7.16~7.14 (m, 2H), 6.97~6.85 (m, 2H), 4.02 (q, J=6.4 Hz, 1H, minor), 3.92 (q, J=6.4 Hz, 1H, major), 3.02~2.96 (m, 1H, major and minor), 2.73~2.62 (m, 2H, major and minor), 2.46~2.33 (m, 2H, major), 2.28~2.16 (m, 2H, minor), 1.53 (s, 9H, major), 1.48 (s, 9H, minor), 1.34 (d, J=6.4 Hz, 3H, major and minor); 13C NMR (100 MHz, CDCl3) δ: 171.1, 156.0 (dd, J=241.1, 7.5 Hz, 1C), 148.2, 146.3, 145.3, 128.1 (2C), 126.8, 126.4 (2C), 122.6, 118.9 (dd, J=18.7, 5.7 Hz, 1C), 104.8 (dd, J=28.7, 20.5 Hz, 1C), 80.6, 55.2, 52.7, 39.2, 33.8, 28.0 (3C), 24.6; 19F NMR (376 MHz, CDCl3) δ: -41.47 (m, 1F), -59.16 (m, 1F), -65.97 (m, 1F). HPLC (X charge C18, V(CH3CN)/ V(buffered solution, CH3COOH and CH3COONa, pH=4.5)=65/35, 1 mL/min, 254 nm): t1=14.9 min, t2=16.4 min.[16]
Compounds 2b~2k were synthesized in a similar way.
(3R)-Isopropyl 3-(((1R)-1-phenylethyl)amino)-4-(2, 4, 5-trifluorophenyl)butanoate (2b): Pale yellow liquid. Yield 76%, dr=13.8:86.2. 1H NMR (400 MHz, CDCl3) δ: 7.28~7.10 (m, 3H), 7.03~7.11 (m, 2H), 6.84~6.72 (m, 2H), 5.00~4.89 (m, 1H, major and minor), 3.89 (q, J=6.8 Hz, 1H, minor), 3.79 (q, J=6.8 Hz, 1H, major), 2.93~2.78 (m, 1H, major and minor), 2.62~2.50 (m, 2H, major and minor), 2.39~2.25 (m, 2H, major), 2.20~2.08 (m, 2H, minor), 1.26~1.10 (m, 9H, major and minor); 13C NMR (100 MHz, CDCl3) δ: 171.4, 157.7, 154.9, 147.7, 145.4, 128.3 (2C), 126.9, 126.5 (2C), 122.5, 119.0 (dd, J=18.6, 5.7 Hz, 1C), 105.0 (dd, J=28.7, 8.0 Hz, 1C), 87.8, 55.3, 52.7, 38.4, 33.9, 24.7, 21.8 (2C); 19F NMR (376 MHz, CDCl3) δ: -41.48 (m, 1F), -59.03 (m, 1F), -65.93 (m, 1F). HRMS-ESI calcd for C21H26F3NO2 (M+ H)+ 380.1837; found 380.1832. HPLC (X charge C18, V(CH3CN)/V(buffered solution, CH3COOH and CH3CO-ONa, pH=4.5)=65/35, 0.7 mL/min, 254 nm): t1=22.0 min, t2=23.7 min.
(3R)-Methyl 3-(((1R)-1-phenylethyl)amino)-4-(2, 4, 5-tri-fluorophenyl)butanoate (2c)[3]: Pale yellow liquid. Yield 79%, dr=14.4:85.6. 1H NMR (400 MHz, CDCl3) δ: 7.41~7.24 (m, 3H), 7.14~7.12 (m, 2H), 6.96~6.85 (m, 2H), 4.01 (q, J=6.8 Hz, 1H, minor), 3.89 (q, J=6.8 Hz, 1H, major), 3.74 (s, 3H, major), 3.66 (s, 3H, minor), 3.07~3.01 (m, 1H, major), 2.98~2.93 (m, 1H, minor), 2.75~2.63 (m, 2H, major and minor), 2.56~2.44 (m, 2H, major), 2.39~2.45 (m, 2H, minor), 1.34 (m, 3H, major and minor); 13C NMR (100 MHz, CDCl3) δ: 172.3, 157.3, 149.7, 147.4, 145.2, 128.3 (2C), 126.9, 126.4 (2C), 122.3, 119.0 (dd, J=18.8, 6.1 Hz, 1C), 105.0 (dd, J=28.6, 20.5 Hz, 1C), 55.4, 52.6, 51.5, 37.9, 33.9, 24.6; 19F NMR (376 MHz, CDCl3) δ: -41.52 (m, 1F), -58.83 (m, 1F), -65.80 (m, 1F). HRMS-ESI calcd for C19H21F3NO2 (M+H)+: 352.1524; found 352.1510. HPLC (X charge C18, V(CH3CN)/V(buffered solution, CH3COOH and CH3CO-ONa, pH=4.5)=65/35, 1 mL/min, 254 nm): t1=15.1 min, t2=16.7 min.
(3R)-tert-Butyl 4-phenyl-3-(((1R)-1-phenylethyl)amino)-butanoate (2d): Pale yellow liquid. Yield 93%, dr=5.9:94.1. 1H NMR (400 MHz, CDCl3) δ: 7.19~7.10 (m, 6H), 6.99~6.97 (m, 4H), 3.93 (q, J=6.8 Hz, 1H, minor), 3.80 (q, J=6.4 Hz, 1H, major), 2.95~2.89 (m, 1H, major), 2.86~2.81 (m, 1H, minor), 2.67~2.54 (m, 2H, major and minor), 2.24 (d, J=6.0 Hz, 2H, major and minor), 1.39 (s, 9H, major), 1.35 (s, 9H, minor), 1.21 (d, J=6.8 Hz, 3H, major and minor); 13C NMR (100 MHz, CDCl3) δ: 171.6, 145.5, 138.8, 129.4 (2C), 128.4 (2C), 128.3 (2C), 126.7, 126.4 (2C), 126.2, 80.4, 55.1, 53.7, 41.3, 39.5, 28.1 (3C), 24.6. HRMS-ESI calcd for C22H20NO2 (M+H)+: 340.2277; found 340.2277. HPLC (X charge C18, V(CH3CN)/V(buffered solution, CH3COOH and CH3CO-ONa, pH=4.5)=65/35, 1 mL/min, 254 nm): t1=15.5 min, t2=16.2 min.
(3R)-tert-Butyl 4-(4-methoxyphenyl)-3-(((1R)-1-pheny-lethyl)amino)butanoate (2e): Pale yellow liquid. Yield 99%, dr=7.4:92.6. 1H NMR (400 MHz, CDCl3) δ: 7.26~7.25 (m, 3H), 7.00~6.97 (d, J=7.6 Hz, 2H), 6.89 (d, J=8.4 Hz, 2H), 6.70 (d, J=8.4 Hz, 2H), 3.91 (q, J=6.4 Hz, 1H, minor), 3.78 (q, J=6.4 Hz, 1H, major), 3.70 (s, 3H, major), 3.68 (s, 3H, minor), 2.90~2.84 (m, 1H, major and minor), 2.61~2.47 (m, 2H, major and minor), 2.22 (d, J=5.6 Hz, 2H, major and minor), 1.38 (s, 9H, major), 1.34 (s, 9H, minor), 1.20 (d, J=6.4 Hz, 3H, major and minor); 13C NMR (100 MHz, CDCl3) δ: 171.6, 158.0, 145.5, 130.7, 130.2 (2C), 128.2 (2C), 126.7, 126.4 (2C), 113.6 (2C), 80.2, 55.1, 55.1, 53.7, 40.3, 39.4, 28.1 (3C), 24.5. HRMS-ESI calcd for C23H32NO3 (M+H)+: 370.2382; found 370.2375. HPLC (X charge C18, V(CH3CN)/V(buffered solution, CH3COOH and CH3CO-ONa, pH=4.5)=65/35, 1 mL/min, 254 nm): t1=15.0 min, t2=16.4 min.
(3R)-tert-Butyl 4-(4-bromophenyl)-3-(((1R)-1-phenyle-thyl) amino)butanoate (2f): Pale yellow liquid. Yield 98%, dr=6.7:93.3. 1H NMR (400 MHz, CDCl3) δ: 7.28~7.25 (m, 2H), 7.18~7.11 (m, 3H), 7.00~6.97 (m, 2H), 6.85~6.83 (m, 2H), 3.92 (q, J=6.4 Hz, 1H, minor), 3.77 (q, J=6.4 Hz, 1H, major), 2.89~2.83 (m, 1H, major and minor), 2.61~2.47 (m, 2H, major and minor), 2.24~2.22 (m, 2H, major and minor), 1.39 (s, 9H, major), 1.35 (s, 9H, minor), 1.19 (d, J=2.0 Hz, 3H, major and minor); 13C NMR (100 MHz, CDCl3) δ: 171.4, 144.8, 137.7, 131.3 (2C), 131.1 (2C), 128.3 (2C), 126.9, 126.5 (2C), 120.0, 80.6, 55.4, 53.7, 40.6, 39.1, 28.1 (3C), 24.4. HRMS-ESI calcd for C22H29BrNO2 (M+H)+: 418.1382; found 418.1384. HPLC (X charge C18, V(CH3CN)/V(buffered solution, CH3CO-OH and CH3COONa, pH=4.5)=65/35, 1 mL/min, 254 nm): t1=15.0 min, t2=15.7 min.
(3R)-tert-Butyl 3-(((1R)-1-phenylethyl)amino)-4-(4-(tri-fluoromethyl)phenyl)butanoate (2g): Pale yellow liquid. Yield 99%, dr=5.7:94.3. 1H NMR (400 MHz, CDCl3) δ: 7.56 (d, J=8 Hz, 2H), 7.28~7.23 (m, 5H), 7.13~7.10 (m, 2H), 4.08 (q, J=6.8 Hz, 1H, minor), 3.94 (q, J=6.8 Hz, 1H, major), 3.09~3.03 (m, 1H, major and minor), 2.88~2.72 (m, 2H, major and minor), 2.47~2.38 (m, 2H, major), 2.29~2.18 (m, 2H, minor), 1.56 (s, 9H, major), 1.52 (s, 9H, minor), 1.36 (d, J=6.8 Hz, 3H, major and minor); 13C NMR (100 MHz, CDCl3) δ: 170.5, 160.5, 145.1, 140.9, 128.8, 127.1, 125.5 (d, J=3.3 Hz), 125.3, 87.4, 78.5, 52.6, 38.8, 28.8, 25.0; 19F NMR (376 MHz, CDCl3) δ: 15.42. HRMS-ESI calcd for C23H29F3NO2 (M+H)+: 408.2150; found 408.2144. HPLC (X charge C18, V(CH3CN)/ V(buffered solution, CH3COOH and CH3COONa, pH=4.5)=65/35, 0.7 mL/min, 254 nm): t1=25.0 min, t2=26.9 min.
(3R)-tert-Butyl 4-(3-chlorophenyl)-3-(((1R)-1-pheny-lethyl) amino)butanoate (2h): Pale yellow liquid. Yield 91%, dr=5.2:94.8. 1H NMR (400 MHz, CDCl3) δ: 7.42~7.02 (m, 9H), 4.06 (q, J=6.4 Hz, 1H, minor), 3.95 (q, J=6.4 Hz, 1H, major), 3.07~3.03 (m, 1H, major and minor), 2.80~2.66 (m, 2H, major and minor), 2.45~2.35 (m, 2H, major), 2.26~2.17 (m, 2H, minor), 1.56 (s, 9H, major), 1.52 (s, 9H, minor), 1.37 (d, J=6.8 Hz, 3H, major and minor); 13C NMR (100 MHz, CDCl3) δ: 171.4, 145.1, 140.9, 134.0, 129.4, 129.4, 128.3 (2C), 127.6, 126.9, 126.5 (2C), 126.4, 80.6, 55.3, 53.5, 41.0, 39.1, 28.1 (3C), 24.5. HRMS-ESI calcd for C22H29ClNO2 (M+H)+: 374.1887; found 374.1883. HPLC (X charge C18, V(CH3CN)/ V(buffered solution, CH3COOH and CH3COONa, pH=4.5)=65/35, 1 mL/min, 254 nm): t1=14.5 min, t2=15.2 min.
(3R)-tert-Butyl 3-(((1R)-1-phenylethyl)amino)-4-(o-tol-yl)butanoate (2i): Pale yellow liquid. Yield 91%, dr=10.1:89.9. 1H NMR (400 MHz, CDCl3) δ: 7.41~7.05 (m, 9H), 4.06 (q, J=6.4 Hz, 1H, minor), 3.93 (q, J=6.8 Hz, 1H, major), 3.12~3.01 (m, 1H, major and minor), 2.85~2.74 (m, 2H, major and minor), 2.48~2.38 (m, 2H, major and minor), 2.19 (s, 3H, minor), 2.15 (s, 3H, major), 1.55 (s, 9H, major), 1.51 (s, 9H, minor), 1.36 (d, J=6.8 Hz, 3H, major and minor); 13C NMR (100 MHz, CDCl3) δ: 171.6, 145.5, 137.0, 136.7, 130.3, 128.3, 128.2 (2C), 126.7, 126.3 (2C), 125.7, 80.4, 55.2, 52.0, 39.8, 39.3, 28.1, 24.6, 19.2. HRMS-ESI calcd for C23H32NO2 (M+H)+: 354.2433; found 354.2429. HPLC (X charge C18, V(CH3CN)/ V(buffered solution, CH3COOH and CH3COONa, pH=4.5)=60/40, 0.7 mL/min, 254 nm): t1=31.3 min, t2=32.6 min.
(3R)-tert-Butyl 4-(2-methoxyphenyl)-3-(((1R)-1-pheny-lethyl)amino)butanoate (2j): Pale yellow liquid. Yield 70%, dr=23.8:76.2. 1H NMR (400 MHz, CDCl3) δ: 7.34~7.16 (m, 5H), 7.06~7.04 (m, 2H), 6.88~6.76 (m, 2H), 4.03 (q, J=6.4 Hz, 1H, minor), 3.87 (q, J=6.4 Hz, 1H, major), 3.67 (s, 3H, minor), 3.63 (s, 3H, major), 3.12~3.06 (m, 1H, major and minor), 2.80~2.65 (m, 2H, major), 2.60~2.54 (m, 2H, minor), 2.36~2.27 (m, 2H, major and minor), 1.46 (s, 9H, major), 1.42 (s, 9H, minor), 1.29 (d, J=6.8 Hz, 3H, major and minor); 13C NMR (100 MHz, CDCl3) δ: 171.9, 157.6, 145.9, 131.2, 128.1 (2C), 127.5, 127.3, 126.5, 126.5 (2C), 120.3, 110.2, 80.2, 55.1, 54.9, 52.4, 40.3, 36.0, 28.1 (3C), 24.3. HRMS-ESI calcd for C23H32NO3 (M+H)+ 370.2382; found 370.2379. HPLC (X charge C18, V(CH3CN)/V(buffered solution, CH3COOH and CH3COONa, pH=4.5)=65/35, 1 mL/ min, 254 nm): t1=13.1 min, t2=14.2 min.
3.2.1 Preparation of 5-(1-hydroxy-2-(2, 4, 5-trifluoro-phenyl)ethylidene)-2, 2-dimethyl-1, 3-dioxane-4, 6-dione (3a) and tert-butyl 3-(((1R)-1-phenylethyl)amino)-4-(2, 4, 5-trifluorophenyl)but-2-enoate (1a)
2, 4, 6-Trifluorophenylacetic acid (10.0 g, 52.6 mmol) was dissolved in dichloromethane (70.0 mL). N, N-Dime-thylformamide (DMF) (4.0 mL) was added subsequently at room temperature. Oxalyl chloride (7.7 g, 60.5 mmol) in dichloromethane (30.0 mL) was slowly added dropwise over a period of 1 h, and stirred at room temperature for 2~3 h until the end of gas evolution. The system was evaporated to remove excess oxalyl chloride. CH2Cl2 (40.0 mL) was added to the residue which could be then slowly added to a mixture of Meldrum's acid (31.8 g, 220.9 mmol) and 2, 6-dimethylpyridine (47.3 g, 441.8 mmol) in CH2Cl2 (100.0 mL) at -5 ℃ for 2 h. The resulting yellow solution was stirred at room temperature for another 2 h. After completion, the reaction was quenched with 10% hydrochloric acid (180.0 mL). Then the layers were sepa-rated, and the organic layer was extracted with 1 mol•L-1NaOH (840.0 mL) twice. The combined aqueous layers were acidified with 37% hydrochloric acid until pH=1. The pale yellow solid 3a[18] was filtered off and dried, which could be used in the next step without further purification.
5-(1-Hydroxy-2-(2, 4, 5-trifluorophenyl)ethylidene)-2, 2-dimethyl-1, 3-dioxane-4, 6-dione (3a) (10.0 g, 31.6 mmol) was dissolved in tert-butanol (50.0 mL), which was subsequently heated to reflux at 85 ℃ for 2 h and then (R)-1-phenylethanamine (4.2 g, 34.8 mmol) and acetic acid (1.9 g, 31.6 mmol) were charged into the system. The mixture was stirred at 40 ℃ overnight. t-BuOH was evaporated under vacuum distillation. The crude product was recrystallized by methanol/water (V:V=95:5, 30.0 mL) to get pure product 1a.[16] The yield of crude product is 89.5%.
(Z)-tert-Butyl 3-(((1R)-1-phenylethyl)amino)-4-(2, 4, 5-trifluorophenyl)but-2-enoate (1a)[16]: White solid. Yield 89.5%. m.p. 71.2~72.5 ℃; [α]D25-395.5 (c 0.25, CHCl3); 1H NMR (400 MHz, CDCl3) δ: 8.89 (d, J=8.0 Hz, 1H), 7.32~7.19 (m, 5H), 7.00~6.93 (m, 1H), 6.88~6.81 (m, 1H), 4.48~4.41 (m, 1H), 4.32 (s, 1H), 3.40 (d, J=16.4 Hz, 1H), 3.15 (d, J=16.4 Hz, 1H), 1.50 (s, 9H), 1.45 (d, J=6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 170.3, 159.4, 155.3 (dd, JC-F=244.8, 11.0 Hz, 1C), 148.8, 146.8, 144.8, 128.8 (2C), 127.0, 125.2 (2C), 120.1, 117.9 (dd, JC-F=19.7, 4.7 Hz), 105.2 (dd, JC-F=28.1, 20.7 Hz), 87.1, 78.5, 52.9, 30.7, 28.5 (3C), 25.0; 19F NMR (376 MHz, CDCl3) δ: -41.50 (m, 1F), -57.71 (m, 1F), -64.73 (m, 1F). HRMS-ESI calcd for C22H25F3NO2 (M+ H)+ 392.1837, found 392.1843.
Compounds 1b~1k were synthesized in a similar way.
(Z)-Isopropyl 3-(((1R)-1-phenylethyl)amino)-4-(2, 4, 5-trifluorophenyl)but-2-enoate (1b): Pale yellow liquid. Yield 90.1%. [α]D25-0.6 (c 0.46, CHCl3); 1H NMR (400 MHz, CDCl3) δ: 9.03 (d, J=8.0 Hz, 1H), 7.33~7.17 (m, 5H), 6.97~6.82 (m, 2H), 5.08~5.02 (m, 1H), 4.52~4.44 (m, 1H), 4.35 (s, 1H), 3.43 (d, J=16.4 Hz, 1H), 3.19 (d, J=16.4 Hz, 1H), 1.46 (d, J=6.8 Hz, 3H), 1.27 (d, J=6.4 Hz, 6H); 13C NMR (100 MHz, CDCl3) δ: 170.1, 160.3, 155.4 (dd, J=243.2, 2.8 Hz, 1C), 149.1 (ddd, J=26.5, 14.3, 12.2 Hz, 1C), 146.9 (ddd, J=15.9, 12.4, 3.5 Hz, 1C), 144.6, 128.8 (2C), 127.2, 125.2 (2C), 119.9 (ddd, J=9.7, 5.3, 4.3 Hz, 1C), 118.0 (dd, J=19.7, 4.4 Hz, 1C), 105.3 (dd, J=28.1, 20.8 Hz, 1C), 85.6, 65.7, 52.7, 30.8, 25.1, 22.2 (2C); 19F NMR (376 MHz, CDCl3) δ: -41.43 (m, 1F), -57.50 (m, 1F), -64.65 (m, 1F). HRMS-ESI calcd for C21H23F3NO2 (M+H)+ 378.1681, found 378.1670.
(Z)-Methyl 3-(((1R)-1-phenylethyl)amino)-4-(2, 4, 5-tri-fluorophenyl)but-2-enoate (1c)[19]: White solid. Yield 84.8%. m.p. 46.4~47.5 ℃; [α]D25+337.5 (c 0.40, CHCl3); 1H NMR (400 MHz, CDCl3) δ: 9.01 (d, J=7.2 Hz, 1H), 7.33~7.17 (m, 5H), 6.96~6.82 (m, 2H), 4.53~4.46 (m, 1H), 4.39 (s, 1H), 3.68 (s, 3H), 3.45 (d, J=16.4 Hz, 1H), 3.21 (d, J=16.4 Hz, 1H), 1.47 (d, J=6.8 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ: 170.7, 160.6, 155.3 (ddd, J=11.8, 9.2 Hz, 2.6 Hz, 1C), 148.8 (ddd, J=26.6, 14.2 Hz, 12.3 Hz, 1C), 146.8 (ddd, J=16.0, 12.5 Hz, 3.6 Hz, 1C), 144, 4, 128.7 (2C), 127.1, 125.1 (2C), 119.8 (ddd, J=9.7, 5.3, 4.4 Hz, 1C), 117.8 (dd, J=19.7, 4.8 Hz, 1C), 105.3 (dd, J=28.1 Hz, 20.8 Hz, 1C), 84.6, 52.7, 50.1, 30.7, 24.9; 19F NMR (376 MHz, CDCl3) δ: -41.39 (m, 1F), -57.37 (m, 1F), -64.55 (m, 1F). HRMS-ESI calcd for C19H19F3NO2 (M+H)+ 350.1368, found 350.1352.
(Z)-tert-Butyl 4-phenyl-3-(((1R)-1-phenylethyl)amino)-but-2-enoate (1d): White solid. Yield 84.3%. m.p. 62.4~ 63.1 ℃; [α]D25-372.5 (c 0.40, CHCl3); 1H NMR (400 MHz, CDCl3) δ: 8.89 (d, J=8.0 Hz, 1H), 7.34~7.14 (m, 10H), 4.48~4.41 (m, 2H), 3.35 (d, J=16.0 Hz, 1H), 3.24 (d, J=15.6 Hz, 1H), 1.50 (s, 9H), 1.38 (d, J=6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 170.6, 161.5, 145.2, 136.9, 128.7 (2C), 128.5 (2C), 128.4 (2C), 126.9, 126.6, 125.3 (2C), 86.8, 78.0, 52.2, 39.0, 28.6 (3C), 24.8. HRMS-ESI calcd for C22H28NO2(M+H)+ 338.2120, found 338.2117.
(Z)-tert-Butyl 4-(4-methoxyphenyl)-3-(((1R)-1-phenyl-ethyl)amino)but-2-enoate (1e): White solid. Yield 86.2%. m.p. 52.1~53.8 ℃; [α]D25-316.6 (c 0.40, CHCl3); 1H NMR (400 MHz, CDCl3) δ: 8.80 (d, J=7.6 Hz, 1H), 7.25~7.10 (m, 5H), 6.96 (d, J=8.0 Hz, 2H), 6.73 (d, J=8.0 Hz, 2H), 4.41~4.34 (m, 1H), 4.31 (s, 1H), 3.69 (s, 3H), 3.20 (d, J=16.0 Hz, 2H), 3.09 (d, J=16.0 Hz, 2H), 1.42 (s, 9H), 1.30 (d, J=6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 170.5, 162.0, 158.2, 145.2, 129.4 (2C), 128.6 (2C), 128.5, 126.8, 125.2 (2C), 113.8 (2C), 86.5, 77.9, 55.0, 52.1, 38.1, 28.6 (3C), 24.8. HRMS-ESI calcd for C23H30NO3(M+H)+ 368.2226, found 368.2217.
(Z)-tert-Butyl 4-(4-bromophenyl)-3-(((1R)-1-phenyle-thyl)amino)but-2-enoate (1f): White solid. Yield 81.2%. m.p. 87.6~88.7 ℃; [α]D25-297.1 (c 0.40, CHCl3); 1H NMR (400 MHz, CDCl3) δ: 8.87 (d, J=8.0 Hz, 1H), 7.40~7.17 (m, 7H), 7.00 (d, J=8.0 Hz, 2H), 4.43~4.36 (m, 2H), 3.29 (d, J=16.0 Hz, 1H), 3.17 (d, J=16.4 Hz, 1H), 1.50 (s, 9H), 1.40 (d, J=6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 170.5, 161.0, 145.2, 135.7, 131.7 (2C), 130.2 (2C), 128.8 (2C), 127.0, 125.3 (2C), 120.5, 87.1, 78.4, 52.4, 38.5, 28.7 (3C), 25.0. HRMS-ESI calcd for C22H27BrNO2(M+H)+ 416.1225, found 416.1223.
(Z)-tert-Butyl 3-(((1R)-1-phenylethyl)amino)-4-(4-(tri-fluoromethyl)phenyl)but-2-enoate (1g): White solid. Yield 80.8%. m.p. 82.2~83.5 ℃; [α]D25-290.6 (c 0.40, CHCl3); 1H NMR (400 MHz, CDCl3) δ: 8.90 (d, J=7.6 Hz, 1H), 7.51 (d, J=8.4 Hz, 2H), 7.33~7.15 (m, 7H), 4.42~4.35 (m, 2H), 3.40 (d, J=16.0 Hz, 1H), 3.29 (d, J=16.0 Hz, 1H), 1.51 (s, 9H), 1.41 (d, J=7.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 170.5, 160.5, 145.1, 140.9, 128.8, 127.1, 125.5 (d, JC-F=3.4 Hz), 125.3, 87.4, 78.5, 52.6, 38.8, 28.6, 25.0; 19F NMR (376 MHz, CDCl3) δ: 15.31. HRMS-ESI calcd for C23H27F3NO2(M+H)+ 406.1994, found 406.2007.
(Z)-tert-Butyl 4-(3-chlorophenyl)-3-(((1R)-1-phenyle-thyl)amino)but-2-enoate (1h): White solid. Yield 80.7%. m.p. 61.2~62.8 ℃; [α]D25-320.1 (c 0.40, CHCl3); 1H NMR (400 MHz, CDCl3) δ: 8.88 (d, J=8.0 Hz, 1H), 7.35~7.02 (m, 9H), 4.43~4.38 (m, 2H), 3.31 (d, J=16.0 Hz, 1H), 3.20 (d, J=16.0 Hz, 1H), 1.51 (s, 9H), 1.41 (d, J=6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 170.5, 160.7, 145.1, 138.7, 134.4, 129.8, 128.8 (2C), 128.5, 127.1, 126.9, 126.7, 125.3 (2C), 87.1, 78.4, 52.4, 38.6, 28.6 (3C), 25.0. HRMS-ESI calcd for C22H27ClNO2 (M+H)+ 372.1730, found 372.1727.
(Z)-tert-Butyl 3-(((1R)-1-phenylethyl)amino)-4-(o-tol-yl)but-2-enoate (1i): White solid. Yield 88.4%. m.p. 84.4~85.2 ℃; [α]D25-15.0 (c 0.40, CHCl3); 1H NMR (400 MHz, CDCl3) δ: 8.99 (d, J=8.0 Hz, 1H), 7.35~7.10 (m, 9H), 4.52~4.47 (m, 1H), 4.18 (s, 3H), 3.39 (d, J=16.4 Hz, 1H), 3.16 (d, J=16.8 Hz, 1H), 2.03 (s, 3H), 1.48~1.47 (m, 12H); 13C NMR (100 MHz, CDCl3) δ: 170.8, 161.8, 145.4, 136.4, 134.8, 130.1, 129.0, 128.7 (2C), 127.0, 126.8, 126.1, 125.4 (2C), 85.9, 78.1, 52.5, 36.3, 28.6 (3C), 25.1, 19.2. HRMS-ESI calcd for C23H30-NO2 (M+H)+ 352.2277, found 352.2270.
(Z)-tert-Butyl 4-(2-methoxyphenyl)-3-(((1R)-1-phenyle-thyl) amino)but-2-enoate (1j): White solid. Yield 85.9%. m.p. 77.9~80.5 ℃; [α]D25-278.6 (c 0.40, CHCl3); 1H NMR (400 MHz, CDCl3) δ: 8.92 (d, J=7.6 Hz, 1H), 7.33~7.14 (m, 7H), 6.90~6.82 (m, 2H), 4.55~4.47 (m, 1H), 4.34 (s, 1H), 4.77 (s, 3H), 3.52 (d, J=16.4 Hz, 1H), 3.14 (d, J=16.4 Hz, 1H), 1.49 (s, 9H), 1.42 (d, J=6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 170.8, 162.3, 156.8, 145.3, 129.5, 128.5 (2C), 127.8, 126.8, 125.6 (2C), 125.1, 120.6, 110.1, 85.9, 78.0, 55.3, 52.3, 32.4, 28.7 (3C), 24.9. HRMS-ESI calcd for C23H30NO3(M+H)+ 368.2226, found 368.2219.
(Z)-tert-Butyl 3-phenyl-3-(((1R)-1-phenylethyl)amino)-acrylate (1k): White solid. Yield 88.0%. m.p. 64.6~65.4 ℃; [α]D25-0.6 (c 0.40, CHCl3); 1H NMR (400 MHz, CDCl3) δ: 8.76 (d, J=9.6 Hz, 1H), 8.78~7.07 (m, 10H), 4.55 (s, 1H), 4.44~4.37 (m, 1H), 1.51 (s, 9H), 1.46 (d, J=6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ: 170.2, 163.5, 145.0, 136.6, 128.8, 128.4 (2C), 128.0 (2C), 127.7 (2C), 126.7, 125.7 (2C), 88.9, 78.5, 53.8, 28.6 (3C), 24.6. HRMS-ESI calcd for C21H26NO2(M+H)+ 324.1964, found 324.1955.
-
-
[1]
(a) Guenard, D. ; Guritte, R. ; Potier, P. Acc. Chem. Res. 1993, 26, 160.
(b) Juaristi, E. Enantioselective Synthesis of β-Amino Acids, Wiley-VCH, New York, 1997.
(c) Seebach, D. ; Abele, S. ; Gademann, K. ; Guichard, G. ; Hintermann, T. ; Jaun, B. ; Matthews, J. ; Schreiber, J. Helv. Chim. Acta 1998, 81, 932.
(d) Seebach, D. ; Matthews, J. Chem. Commun. 1997, 21, 2015.
(e) Liu. M. ; Sibi, M. Tetrahedron 2002, 58, 7991.
(f) Davies, H. ; Venkataramani, C. Angew. Chem. , Int. Ed. 2002, 41, 2197.
(g) Cole, D. Tetrahedron 1994, 50, 9517. -
[2]
(a) Kim, D. ; Wang, L. ; Beconi, M. J. Med. Chem. 2004, 48, 141.
(b) Hansen, K. B. ; Balsells, J. ; Dreher, S. ; Hsiao, Y. ; Kubryk, M. ; Palucki, M. ; Rivera, N. R. ; Steinhuebel, D. ; Armstrong, J. D. ; Askin, D. ; Grabowski, E. Org. Process Res. Dev. 2005, 9, 634. -
[3]
(a) Porter, E. A. ; Wang, X. ; Lee, H. ; Weisblum, B. ; Gellman, S. Nature 2000, 404, 565.
(b) Wang, X. ; Espinosa, J. F. ; Gellman, S. H. J. Am. Chem. Soc. 2000, 122, 4821.
(c) Appella, D. H. ; Christianson, L. A. ; Klein, D. A. ; Richards, M. R. ; Powell, D. R. ; Gellman, S. H. J. Am. Chem. Soc. 1999, 121, 7574.
(d) Drexler, H. J. ; You, J. ; Zhang, S. ; Fischer, C. ; Baumann, W. ; Spannenberg, A. ; Heller, D. Org. Process Res. Dev. 2003, 7, 355. -
[4]
(a) Jefford, C. W. ; McNulty, J. ; Lu, Z. H. ; Wang, J. B. Helv. Chim. Acta 1996, 79, 1203.
(b) Shimamoto, K. ; Ishida, M. ; Shinozaki, H. ; Ohfune, Y. J. Org. Chem. 1991, 56, 4167.
(c) Juaristi, E. ; Quintana, D. ; Balderas, M. ; Garcia, P. Tetrahedron: Asymmetry 1996, 7, 2233.
(d) Sleebs, B. E. ; Hughes, A. B. J. Org. Chem. 2007, 72, 3340. -
[5]
Vicario, J. L.; Badia, D.; Carrillo, L. Org. Lett. 2001, 3, 773. doi: 10.1021/ol0155384
-
[6]
(a) Dreher, S. D. ; Ikemoto, N. ; Njolito, E. ; Rivera, N. R. ; Tellers, D. M. ; Xiao, Y. WO 2004085661, 2004[Chem. Abstr. 2004, 141, 332476].
(b) Karl, B. H. ; Jaume, B. ; Spencer, D. ; Yi, H. ; Joseph, D. A. ; David, A. ; Edward, J. J. G. Org. Process. Res. Dev. 2005, 9, 634. -
[7]
(a) Liu, F. ; Yu, W. ; Ou, W. ; Wang, X. ; Ruan, L. ; Li, Y. ; Peng, X. ; Tao, X. ; Pan, X. J. Chem. Res. 2010, 34, 230.
(b) Guichard, G. ; Abelem, S. ; Seebach, D. Helv. Chim. Acta 1998, 81, 187.
(c) Faulconbridge, S. J. ; Holy, K. E. ; Sevillano, L. G. ; Lock, C. J. ; Tiffin, P. D. ; Tremayne, N. ; Winter, S. Tetrahedron Lett. 2000, 41, 2679. -
[8]
(a) Dumas, F. ; Mezrhab, B. ; Angelo, J. ; Riche, C. ; Chiaroni, A. J. Org. Chem. 1996, 61, 2293.
(b) Kobayashi, S. ; Ishitani, H. ; Ueno, M. J. Am. Chem. Soc. 1998, 120, 431.
(c) Tang, T. P. ; Ellman, J. A. J. Org. Chem. 1999, 64, 12.
(d) Zhou, Y. ; Tang, W. ; Wang, W. ; Li, W. ; Zhang, X. J. Am. Chem. Soc. 2002, 124, 4952.
(e) Tang, W. ; Wang, W. ; Chi, Y. ; Zhang, X. Angew. Chem. , Int. Ed. 2003, 42, 3509. -
[9]
(a) Bruneau, C. ; Renaud, J. ; Jerphagnon, T. Coord. Chem. Rev. 2008, 252, 532.
(b) Vineyard, B. D. ; Knowles, W. S. ; Sabacky, M. J. ; Bachman, G. L. ; Weinkauff, D. J. J. Am. Chem. Soc. 1977, 99, 5946.
(c) Achiwa, K. ; Soga, T. Tetrahedron Lett. 1978, 13, 1119.
(d) Lubell, W. D. ; Kitamura, M. ; Noyori, R. Tetrahedron: Asymmetry 1991, 2, 543.
(e) Zhu, G. ; Chen, Z. ; Zhang, X. J. Org. Chem. 1999, 64, 6907.
(f) Heller, D. ; Holz, J. ; Drexler, H. ; Lang, J. ; Drauz, K. ; Krimmer, H. ; Börner, A. J. Org. Chem. 2001, 66, 6816.
(g) Yasutake, M. ; Gridnev, I. D. ; Higashi, N. ; Imamoto, T. Org. Lett. 2001, 3, 1701.
(h) Heller, D. ; Drexler, H. ; You, J. ; Baumann, W. ; Drauz, K. ; Krimmer, H. ; Börner, A. Chem. Eur. J. 2002, 8, 5196.
(i) Zhou, Y. G. ; Tang, W. ; Wang, W. B. ; Li, W. ; Zhang, X. J. Am. Chem. Soc. 2002, 124, 4952.
(j) Yang, W. ; Wu, S. ; Zhang, X. J. Am. Chem. Soc. 2003, 125, 9570.
(k) Komarov, I. V. ; Monsees, A. ; Spannenberg, A. ; Baumann, W. ; Schmidt, U. ; Fischer, C. ; Börner, A. Eur. J. Org. Chem. 2003, 138.
(l) You, J. ; Drexler, H. J. ; Zhang, S. ; Fischer, C. ; Heller, D. Angew. Chem. , Int. Ed. 2003, 115, 942.
(m) You, J. ; Drexler, H. J. ; Zhang, S. ; Fischer, C. ; Heller, D. Angew. Chem. , Int. Ed. 2003, 42, 913.
(n) Wu, J. ; Chen, X. ; Guo, R. ; Yeung, C. H. ; Chan, A. J. Org. Chem. 2003, 68, 2490.
(o) Wu, H. P. ; Hoge, G. Org. Lett. 2004, 6, 3645.
(p) Hsiao, Y. ; Rivera, N. R. ; Rosner, T. ; Krska, S. W. ; Njolito, E. ; Wang, F. ; Sun, Y. ; Armstrong, J. D. ; Grabowski, E. J. J. ; Tillyer, R. D. ; Spindler, F. ; Malan, C. J. Am. Chem. Soc. 2004, 126, 9918.
(q) Dai, Q. ; Yang, W. ; Zhang, X. X. Org. Lett. 2005, 7, 5343.
(r) Tang, W. J. ; Zhang, X. X. Org. Lett. 2002, 4, 4159.
(s) Wang, Q. S. ; Xie, J. H. ; Li, W. ; Zhu, S. F. ; Wang, L. X. ; Zhou, Q. L. Org. Lett. 2011, 13, 3388.
(t) Zhou, X. M. ; Huang, J. D. ; Luo, L. B. ; Zhang, C. L. ; Zhang, Z. ; Hu, X. P. Tetrahedron: Assymmetry 2010, 21, 420.
(u) Pignataro, L. ; Bovio, C. ; Civera, M. ; Piarulli, U. ; Gennari, C. Chem. Eur. J. 2012, 18, 10368.
(v) Lyubimov. S. E. ; Rastorguev. E. A. ; Davankov. V. A. Chirality 2011, 23, 624.
(w) Gridnev, I. D. ; Liu, Y. Y. ; Imamoto, T. ACS Catal. 2014, 4, 203-219. -
[10]
Irfan, M.; Glasnov, T. N.; Kappe, C. O. ChemSusChem 2011, 4, 300. doi: 10.1002/cssc.v4.3
-
[11]
(a) Augustine, R. ; Tanielyan, S. ; Anderson, S. ; Yang, H. Chem. Commun. 1999, 13, 1257.
(b) Sun, Q. ; Meng, X. ; Liu, X. ; Zhang, X. ; Yang, Y. ; Yang, Q. ; Xiao, F. S. Chem. Commun. 2012, 48, 10505. -
[12]
(a) Mallat, T. ; Orglmeister, E. ; Baiker, A. Chem. Rev. 2007, 107, 4863.
(b) Bartók, M. Chem. Rev. 2010, 110, 1663.
(c) Ren, X. M. ; Kong, S. N. ; Shu, Q. D. ; Shu, M. H. Chin. J. Chem. 2016, 34, 373.
(d) Xu, G. L. ; Gang, F. L. ; Dong, T. S. ; Fu, Y. ; Du, Z. Y. Chin. J. Org. Chem. 2016, 36, 1513 (in Chinese).
(徐光利, 刚芳莉, 董涛生, 傅颖, 杜正银, 有机化学, 2016, 36, 1513. )
(e) Bi, C. ; Xiong, X. Q. ; Shi, L. ; Xiao, S. Y. Chin. J. Org. Chem. 2016, 36, 1847 (in Chinese).
(毕成, 熊兴泉, 石霖, 肖上运, 有机化学, 2016, 36, 1847).
(f) Xiao, L. W. ; Peng, X. X. ; Zhou, Q. X. ; Kou, W. ; Shi, Y. R. Chin. J. Org. Chem. 2015, 35, 1204 (in Chinese).
(肖立伟, 彭晓霞, 周秋香, 寇伟, 时亚茹, 有机化学, 2015, 35, 1204. )
(g) Huang, G. ; Chen, Y. Z. ; Jiang, H. L. Acta Chim. Sinica 2016, 74, 113 (in Chinese).
(黄刚, 陈玉贞, 江海龙, 化学学报, 2016, 74, 113. )
(h) Wang, C. A. ; Wang, W. Acta Chim. Sinica 2015, 73, 498 (in Chinese).
(王昌安, 王为, 化学学报, 2015, 73, 498. ) -
[13]
(a) Borszeky, K. ; Mallat, T. ; Baiker, A. Catal. Lett. 1996, 41, 199.
(b) Tungler, A. ; Sipos, E. ; Hada, V. Curr. Org. Chem. 2006, 10, 1569.
(c) Szöllösi, G. ; Varga, T. ; Felföldi, K. ; Cserényi, S. ; Bartók, M. Catal. Commun. 2008, 10, 421. -
[14]
(a) Zsigmond, A. ; Balatoni, I. ; Notheisz, F. ; Hegedüs, C. ; Bakos, J. Catal. Lett. 2005, 101, 195.
(b) Coulston, N. J. ; Jeffery, E. L. ; Wells, R. K. ; Mcmorn, P. ; Wells, P. B. ; Willock, D. J. ; Hutchings, G. J. J. Catal. 2006, 243, 360.
(c) Coulston, N. J. ; Wells, R. P. K. ; Wells, P. B. ; Hutchings, G. J. Catal. Today 2006, 114, 353.
(d) Szöllösi, G. ; Szabo, E. ; Bartók, M. Adv. Synth. Catal. 2007, 349, 405.
(e) Shi, L. ; Wang, X. W. ; Sandoval, C. ; Wang, Z. ; Wu, L. ; Yu, L. Y. ; Ding, K. L. Chem. Eur. J. 2009, 15, 9855. -
[15]
Ikemoto, N.; Tellers, D. M.; Dreher, S. D.; Liu, J. C.; Huang, A.; Rivera, N. R.; Njolito, E.; Hsiao, Y.; McWilliams, J. C.; Williams, J. M.; Armstrong, J. D.; Sun, Y. K.; Mathre, D. J.; Grabowski, E. J. J.; Tillyer, K. D. J. Am. Chem. Soc. 2004, 126, 3048. doi: 10.1021/ja038812t
-
[16]
Jadav, K. J. ; Bhatt, R. M. ; Borkhataria, K. N. ; Chitturi, T. R. ; Thennati, R. WO 2011135586, 2011 [Chem. Abstr. 2011, 155, 615344].
-
[17]
Chen, C. H.; Zhan, E.; Li, Y.; Shen, W. J. J. Mol. Catal. A-Chem. 2013, 379, 117. doi: 10.1016/j.molcata.2013.08.004
-
[18]
(a) Angeland, R. ; Zhong, Y. L. ; Maligres, P. ; Lee, J. ; Askin, D. J. Org. Chem. 2005, 70, 1949.
(b) Wang, H. B. ; Zhou, Y. F. ; Qiu, L. H. ; Yao, R. W. ; Zheng, Y. ; Zhang, C. ; Bao, H. G. ; Xu. X. F. Adv. Synth. Catal. 2016, 358, 1571. -
[19]
Yuan, J. D. ; Xu, A. T. CN 102126976, 2011[Chem. Abstr. 2011, 155, 212107].
-
[1]
-
Table 1. Optimization of catalysts and additivesa

Table 2. Optimization of the reaction solventsa

Table 3. Optimization of hydrogenation pressure and temperaturea

Table 4. Asymmetric hydrogenation of β-dehydroamino estersa

-

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 3
- 文章访问数: 1377
- HTML全文浏览量: 101


 下载:
下载:


 下载:
下载:

