 图式1
之前的研究和本工作
图式1.
Previous studies and this work
图式1
之前的研究和本工作
图式1.
Previous studies and this work

Citation: Lin Songbo, He Xingrui, Meng Jinpeng, Gu Haining, Zhang Peizhi, Wu Jun. Transition-Metal-Free Synthesis of o-Halodiarylamines from o-Halophenols[J]. Chinese Journal of Organic Chemistry, 2017, 37(7): 1864-1869. doi: 10.6023/cjoc201701012

无过渡金属存在下由邻卤苯酚合成邻卤二芳胺
-
关键词:
- 无过渡金属反应
- / 邻卤苯酚
- / 邻卤二芳胺
- / Smiles重排反应
English
Transition-Metal-Free Synthesis of o-Halodiarylamines from o-Halophenols
-
由于邻卤二芳胺可以作为许多天然产物、功能分子和药物的关键前体化合物, 因此邻卤二芳胺的合成受到了有机化学家的关注[1].如Scheme 1所示, 二芳胺的合成主要有两个途径:一是钯催化的卤代苯与苯胺的偶联反应(Buchwald-Hartwig reaction)[2]; 另一种是铜催化的苯硼酸与苯胺的偶联反应(Chan-Lam coupling)[3].然而用上述方法在合成邻卤二芳胺的时候遇到了一些困难, 因为碳-卤键在过渡金属催化的条件下较敏感, 易发生副反应.此外, 过渡金属通常比较昂贵,并且对人体和环境有一定的负面影响[4].因此, 通过非过渡金属的方法实现邻卤二芳胺的合成就变得尤为迫切.
近些年, 非过渡金属的方法实现邻卤二芳胺的研究取得了显著的进展. 2003年, Larock小组[5]报道了从芳基甲磺酸酯出发, 通过苯炔中间体建立了一种非过渡金属催化N-芳基类化合物合成邻卤二芳胺的方法. 2007年, Wood小组[6]报道了用二芳基碘鎓盐构建二芳胺的方法. 2012年Rodrigues等[7]用碘使2-环己基酮胺基化和芳构化来构建邻碘二芳胺.然而,这些非过渡金属的方法都需要相对昂贵的原料, 并且芳环的多样性也很有限.因此, 高效、通用、易操作地合成邻卤二芳胺类化合物依然存在着挑战.本文报道了一种从邻卤苯酚出发, 在非过渡金属存在下合成邻卤二芳胺类化合物的新方法.
1 结果与讨论
1.1 反应条件的选择
我们小组[8]之前报道了一种非金属合成简单二芳胺化合物的方法.本文设想通过反应条件的优化从邻卤苯酚出发合成邻卤二芳胺类化合物.为此, 我们先在50 ℃条件下, 以邻碘苯酚(1a)和2-溴-N-(4-甲氧苯基)丙酰胺(2a)为起始原料合成了中间产物2-(2-碘苯氧基)-N-(4-甲氧苯基)丙酰胺(3a) (Eq. 1).然后, 以3a作为模型化合物, 进行反应条件的筛选和优化, 实验结果如表 1所示.以KOH为碱[8]可获得目标产物4a, 但收率只有26%, 同时得到副产物4ab, 收率为33%(表 1, Entry 1).试用碱性较弱的Cs2CO3或者K2CO3, 转化率却急剧下降(表 1, Entries 2, 3).但试用更强的碱KOtBu可以提高转化率, 但没有分离到3a且只以46%的收率得到了副产物4ab (表 1, Entry 4).加倍增加KOH, 也没有显著地提高产物的收率(表 1, Entry 5).设想能否改变碱的加入方式来提高收率, 我们在130 ℃下分两次等量加入KOH, 反应仍然不理想(表 1, Entry 6).尝试微波加热也没有得到良好的结果(表 1, Entry 7).最后,我们发现加入1.2 equiv.的KOH后在130℃反应2 h, 然后降低温度至50 ℃后再加入等量的KOH, 升温至130 ℃反应2 h.这种改进的方法获得了良好的结果, 以66%的收率得到了目标产物3a(表 1, Entry 8).

Entry Base Base/equiv. Time/h Conv./% Yieldb/% 4a 4ab 1 KOH 1.2 2 57 26 33 2 Cs2CO3 1.2 12 38 24 13 3 K2CO3 1.2 12 0 0 0 4 KOtBu 1.2 2 >99 0 46 5 KOH 2.4 4 — 30c — 6d KOH 1.2+1.2 2+2 — 33c — 7e KOH 1.2+1.2 1+1 — 28c — 8f KOH 1.2+1.2 2+2 — 66c — a Reagents and conditions: 3a(0. 2 mmol), base (0. 24 mmol) and DMSO (0.6 mL) were heated from room temperature to 130 ℃; bDetermined by 1H NMR of the crude reaction mixture; cIsolated yield; dThe base was added by two batches evenly each time at 130 ℃; eThe reaction mixture was heated by microwave and the base was added by two batches evenly each time; fThe second dose base was added after the mixture was cooled to 50 ℃. 1.2 底物拓展
为了考察该合成方法的普遍适用性, 在上述最佳反应条件下进行了底物拓展研究(表 2).首先, 我们考察了2-碘苯酚与2-溴-N-(芳基)丙酰胺(2)的反应.当苯环的对位是供电子基(如甲氧基和甲基)时, 反应能顺利进行, 以较好的产率得到目标产物4a和4c.当苯环的对位是吸电子基团(如氟、氯和溴)时, 产物4d、4e和4f的产率相对较低.当苯环的邻位是甲基时, 没有得到目标产物4g.而苯环的间位为甲基时, 以53%的产率得到目标产物4h.然后, 我们考察了2-溴取代苯酚与2-溴-N-(芳基)丙酰胺2的反应.无论2-溴-N-(芳基)丙酰胺带有供电子基(如甲氧基和甲基), 还是带有吸电子基团(如氟和氯)都可以和4-甲基-2-溴苯酚反应, 以中等产率得到产物4j、4k、4l和4m.同时也考察了其它4-取代-2-溴苯酚参与的反应, 分别得到了相应的产物4n和4o.有趣的是, 1-溴-2-萘酚也可以与2-溴-N-(甲氧苯基)丙酰胺(2a)反应, 以38%的产率得到了产物4p.



a Reagents and conditions: (1) o-Halophenols 1(1. 0 mmol), 2-bromo-N-arylpropanamide 2(1.2 mmol), KOH (1.2 mmol), DMSO (3 mL), 50 ℃, 2 h; (2) KOH (1.2 mmol), 130 ℃, 2 h; (3) KOH (1.2 mmol) was added after the mixture was cooled to 50 ℃, then the mixture was heated to 130 ℃ again and stirred for 2 h; isolated yields were given. 1.3 可能的反应机理
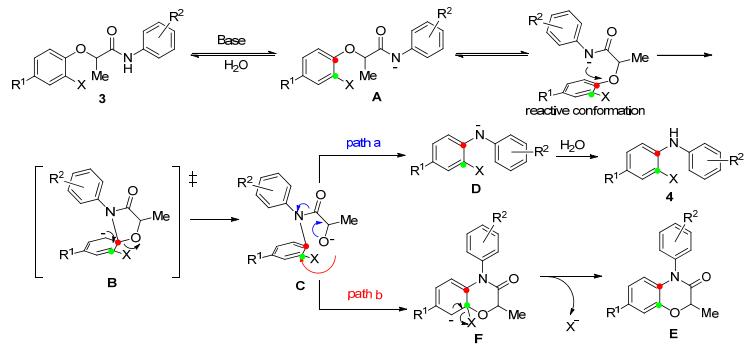
基于上述实验结果, 我们提出了可能的反应机理(Scheme 2)[9]. 第一步是3在强碱作用下通过去质子化生成酰胺负离子A[10].负离子A进攻芳环形成五元环状过渡态B[11], B进而生成中间体C.从3到关键中间体C的转化是一种典型的碱催化的Smiles重排反应.从中间体C通过途径a得到苯胺负离子D, 进一步生成最终产物4, 这一步涉及到酰胺键的C—N键断裂.含氧负离子C也可通过途径b, 进攻苯环得到中间体F, 然后F中的卤素负离子离去最终生成副产物E[12].
2 结论
我们发展了一种KOH/DMSO促进的由邻卤苯酚为原料经Smiles重排反应合成邻卤二苯胺的方法.这种绿色、简便的合成方法在有机合成化学和其它方面将得到很好的应用.
3 实验部分
3.1 仪器与试剂
WRR熔点仪(上海申光仪器仪表有限公司, 温度未经校正); Bruker Advance-400 FT型核磁共振仪(德国Bruker公司, 溶剂为CDCl3, TMS为内标); GCT Premier气相色谱高分辨飞行时间质谱联用仪(美国WATERS公司); NICOLET iS10傅里叶红外光谱仪(美国Thermo Fisher Scientific公司); 紫外灯检测(UV-8三用紫外仪); 薄层色谱用硅胶(GF254, 青岛海洋化工厂产品).其余试剂均为国产化学纯或分析纯.
3.2 实验方法
3.2.1 2-溴-N-(芳基)丙酰胺2的合成
向100 mL反应瓶中先后加入芳胺(20 mmol)、2-溴丙酸(21 mmol)和30 mL二氯甲烷, 搅拌溶解, 加入4-二甲基吡啶(DMAP, 0.2 mmol).将反应混合物置于0 ℃冰水浴中, 分批加入二环己基碳二亚胺(DCC, 21 mmol), 搅拌过夜.反应液用硅胶抽滤后, 滤液旋干.用乙醇重结晶后, 得2-溴-N-(芳基)丙酰胺2, 直接用于下一步反应.
3.2.2 邻卤二芳胺的合成
向25 mL反应瓶中加入邻卤苯酚1(1.0 mmol)、2-溴-N-(芳基)丙酰胺2 (1.2 mmol)、KOH (1.2 mmol)和溶剂DMSO (3 mL), 在50 ℃下搅拌反应2 h, 然后再加入KOH (1.2 mmol), 加热到130 ℃后继续搅拌反应2 h.反应温度降至50 ℃, 再加入KOH (1.2 mmol), 升温到130 ℃继续搅拌反应2 h.冷却, 用10 mL的饱和氯化钠溶液淬灭反应, 乙酸乙酯(10 mL×3) 萃取, 无水Na2SO4干燥, 过滤, 将滤液旋蒸, 剩余物质用硅胶柱层析(乙酸乙酯/石油醚作展开剂梯度洗脱)分离得到邻卤二芳胺4.
2-碘-N-(4-甲氧苯基)苯胺(4a):黄色液体, 产率66%. 1H NMR (400 MHz, CDCl3) δ: 7.71 (dd, J=7.9, 1.4 Hz, 1H), 7.17~7.06 (m, 3H), 6.93~6.83 (m, 3H), 6.52 (td, J=7.8, 1.5 Hz, 1H), 5.78 (s, 1H), 3.81 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 156.48, 145.81, 139.45, 134.61, 129.20, 124.70, 120.63, 114.88, 113.87, 86.71, 55.68; IR (KBr) ν: 3375, 3060, 2998, 2952, 2832, 1583, 1511, 1450, 1398, 1295, 1245, 1180, 1106, 1036, 1007, 817, 744, 612 cm-1; HRMS (EI-TOF) calcd for C13H12NOI (M+) 324.9964, found 324.9969.
2-碘-N-苯基苯胺(4b)[13]:黄色油状液体, 产率67.3%. 1H NMR (400 MHz, CDCl3) δ: 7.80~7.73 (m, 1H), 7.35~7.26 (m, 2H), 7.22~7.08 (m, 4H), 7.07~6.98 (m, 1H), 6.61 (ddd, J=8.0, 5.4, 3.4 Hz, 1H), 5.90 (s, 1H); 13C NMR (100 MHz, CDCl3) δ: 144.03, 142.10, 139.65, 129.59, 129.16, 122.67, 122.04, 120.11, 115.95, 88.89; IR (KBr) ν: 3381, 3058, 1590, 1578, 1505, 1461, 1446, 1413, 1310, 1284, 1218, 1175, 1159, 1077, 1008, 882, 744, 693, 636, 574 cm-1.
2-碘-N-(对甲苯基)苯胺(4c)[14]:棕色液体, 产率81%. 1H NMR (400 MHz, CDCl3) δ: 7.74 (dd, J=7.9, 1.4 Hz, 1H), 7.20~7.01 (m, 6H), 6.57 (td, J=7.9, 1.6 Hz, 1H), 5.85 (s, 1H), 2.33 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 144.74, 139.56, 139.27, 132.79, 130.14, 129.16, 121.34, 121.29, 114.99, 87.94, 20.97;IR (KBr) ν: 3381, 2918, 1587, 1515, 1450, 1396, 1309, 1219, 1109, 1008, 806, 743 cm-1.
2-碘-N-(4-氟苯基)苯胺(4d):无色液体, 产率27%. 1H NMR (400 MHz, CDCl3) δ: 7.75 (dd, J=8.0, 1.7 Hz, 1H), 7.17 (ddd, J=8.7, 7.2, 1.6 Hz, 1H), 7.11 (dd, J=9.3, 4.9 Hz, 3H), 7.07~6.94 (m, 6H), 6.64~6.55 (m, 1H); 13C NMR (100 MHz, CDCl3) δ: 159.13 (d, J=240.7 Hz), 144.80, 139.64, 137.93, 129.27, 123.27 (d, JC-F=8.0 Hz), 121.64, 116.31 (d, JC-F=22.4 Hz), 114.85, 87.86; IR (KBr) ν: 3381, 3059, 1604, 1583, 1506, 1453, 1395, 1310, 1212, 1154, 1096, 1008, 820, 783, 744 cm-1. HRMS (EI-TOF) calcd for C12H9NIF 312.9764, found 312.9764.
2-碘-N-(4-氯苯基)苯胺(4e):黄色油状液体, 产率32%. 1H NMR (400 MHz, CDCl3) δ: 7.78 (dd, J=7.9, 1.4 Hz, 1H), 7.30~7.24 (m, 5H), 7.21 (ddd, J=8.5, 7.2, 1.4 Hz, 1H), 7.14 (dd, J=8.2, 1.6 Hz, 1H), 7.09~7.00 (m, 2H), 6.69~6.60 (m, 1H), 5.86 (s, 1H); 13C NMR (100 MHz, CDCl3) δ: 143.63, 140.86, 139.76, 129.59, 129.28, 127.35, 122.60, 121.08, 116.32, 89.28; IR (KBr) ν: 3380, 1585, 1493, 1453, 1310, 1175, 1091, 1010, 813, 747 cm-1. HRMS (EI-TOF) calcd for C12H9NClI 328.9467, found 328.9468.
2-碘-N-(4-溴苯基)苯胺(4f):棕色液体, 产率36%. 1H NMR (400 MHz, CDCl3) δ: 7.78 (dd, J=7.9, 1.3 Hz, 1H), 7.44~7.35 (m, 2H), 7.25~7.14 (m, 2H), 7.03~6.95 (m, 2H), 6.72~6.62 (m, 1H), 5.85 (s, 1H); 13C NMR (100 MHz, CDCl3) δ: 143.44, 141.45, 139.78, 132.52, 129.28, 122.78, 121.21, 116.60, 114.58, 89.54; IR (KBr) ν: 2932, 1715, 1580, 1506, 1464, 1355, 1294, 1245, 1178, 1124, 1032, 915, 835, 684 cm-1. HRMS (EI-TOF) calcd for C12H9NBrI 372.8962, found 372.8963.
2-碘-N-(间甲苯基)苯胺(4h):黄色液体, 产率53%. 1H NMR (400 MHz, CDCl3) δ: 7.80~7.73 (m, 1H), 7.23~7.15 (m, 3H), 6.94 (d, J=6.5 Hz, 2H), 6.89~6.82 (m, 1H), 6.65~6.56 (m, 1H), 2.33 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 143.98, 141.89, 139.49, 139.42, 129.27, 129.02, 123.41, 121.77, 120.68, 117.01, 115.88, 88.68, 21.49; IR (KBr) ν: 3382, 3035, 2918, 2857, 1693, 1607, 1579, 1510, 1488, 1462, 1441, 1406, 1311, 1249, 1167, 1113, 1089, 1043, 1008, 941, 743, 692, 668, 650 cm-1. HRMS (EI-TOF) calcd for C13H12NI 309.0015, found 309.0012.
2-溴-N-苯基苯胺(4i)[14]:黄色油状液体, 产率75%. 1H NMR (400 MHz, CDCl3) δ: 7.52 (dd, J=8.0, 1.4 Hz, 2H), 7.37~7.29 (m, 4H), 7.25 (dd, J=6.8, 1.3 Hz, 2H), 7.20~7.11 (m, 6H), 7.09~6.99 (m, 2H), 6.73 (ddd, J=8.0, 7.3, 1.6 Hz, 2H), 6.08 (s, 1H); 13C NMR (100 MHz, CDCl3) δ: 141.72, 133.11, 129.59, 128.23, 122.83, 121.03, 120.39, 115.91, 112.30;IR (KBr) ν: 3396, 3062, 3040, 1592, 1505, 1464, 1450, 1415, 1313, 1239, 1219, 1176, 1158, 1123, 1078, 1044, 1021, 884, 743, 694, 666, 637, 575 cm-1.
2-溴-N-(4-甲氧基苯基)-4-甲基苯胺(4j):黄色液体, 产率57%. 1H NMR (400 MHz, CDCl3) δ: 7.32 (s, 1H), 7.25 (s, 0H), 7.09 (d, J=8.8 Hz, 2H), 6.95~6.84 (m, 4H), 5.79 (s, 1H), 3.80 (s, 3H), 2.24 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 156.05, 140.72, 134.98, 133.15, 129.69, 128.87, 123.87, 114.81, 114.69, 111.01, 77.48, 77.16, 76.84, 55.69, 20.28; IR (KBr) ν: 3392, 2999, 2954, 2925, 2833, 1595, 1513, 1455, 1441, 1400, 1315, 1295, 1244, 1180, 1107, 1044, 1107, 1044, 1020, 838, 817, 770, 743, 612, 551 cm-1. HRMS (EI-TOF) calcd for C14H14NI 323.0171, found 323.0168.
2-溴-N-(对甲苯基)4-甲基苯胺(4k):黄色液体, 产率53%. 1H NMR (400 MHz, CDCl3) δ: 7.34 (d, J=1.5 Hz, 1H), 7.24 (s, 1H), 7.14~7.07 (m, 2H), 7.01 (d, J=8.4 Hz, 2H), 6.94 (dd, J=8.3, 1.6 Hz, 1H), 5.87 (s, 1H), 2.31 (s, 3H), 2.25 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 139.64, 139.54, 133.26, 132.08, 130.52, 130.06, 128.84, 120.43, 115.96, 112.12, 77.48, 77.16, 76.84, 20.90, 20.35; IR (KBr) ν: 3399, 3024, 2918, 2852, 1605, 1515, 1387, 1312, 1277, 1225, 1117, 1102, 1037, 869, 808, 697, 669 cm-1. HRMS (EI-TOF) calcd for C14H14NBr 275.0310, found 275.0310.
2-溴-N-(4-氟苯基)-4-甲基苯胺(4l):黄色液体, 产率55%. 1H NMR (400 MHz, CDCl3) δ: 7.37~7.32 (m, 1H), 7.10~7.03 (m, 2H), 7.03~6.92 (m, 4H), 5.81 (s, 1H), 2.25 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 158.73 (d, J=239.9), 139.56, 138.27 (d, JC-F=26.0), 133.34, 130.88, 128.93, 122.26 (d, JC-F=39.0), 116.28, 115.93 (d, JC-F=25.5), 112.12, 77.48, 77.16, 76.84, 20.35; IR (KBr) ν: 3401, 3035, 2920, 2855, 1608, 1514, 1505, 1386, 1314, 1277, 1214, 1155, 1096, 1038, 1012, 996, 870, 834, 815, 699, 670, 592 cm-1.HRMS (EI-TOF) calcd for C13H11NFBr 279.0059, found 279.0060.
2-溴-N-(4-氯苯基)-4-甲基苯胺(4m):白色固体, 产率50%. m.p. 63.7~64.6 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.40~7.35 (m, 1H), 7.26~7.21 (m, 2H), 7.12 (d, J=8.3 Hz, 1H), 7.04~6.97 (m, 3H), 2.28 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 141.26, 138.32, 133.50, 132.01, 129.50, 128.96, 126.66, 120.22, 117.37, 113.39, 20.44; IR (KBr) ν: 3391, 3053, 3020, 2920, 1891, 1605, 1590, 1507, 1489, 1428, 1387, 1308, 1274, 1223, 1148, 1084, 1035, 1013, 857, 825, 810, 717, 699, 545 cm-1. HRMS (EI-TOF) calcd for C13H11NClBr 294.9763, found 294.9767.
2-溴-N-(4-甲氧苯基)-4-氯苯胺(4n):黄色固体, 产率23%. m.p. 83.4~85.1 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.39~7.34 (m, 1H), 7.27~7.18 (m, 2H), 7.12 (d, J=8.3 Hz, 1H), 7.04~6.95 (m, 3H), 2.27 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 141.25, 138.32, 133.49, 131.99, 129.48, 128.95, 126.65, 120.20, 117.37, 113.40, 20.43; IR (KBr) ν: 3394, 2965, 2932, 2836, 1588, 1561, 1510, 1494, 1486, 1463, 1442, 1414, 1389, 1316, 1290, 1266, 1257, 1239, 1217, 1166, 1102, 1030, 862, 820, 806, 773, 697, 669, 584 cm-1. HRMS (EI-TOF) calcd for C13H11NClBr 310.9713, found 310.9709.
2-溴-N-(4-甲氧苯基)-4-叔丁基苯胺(4o):黄色液体, 产率64%. 1H NMR (400 MHz, CDCl3) δ: 7.49 (d, J=2.2 Hz, 1H), 7.17~7.06 (m, 3H), 6.95~6.84 (m, 3H), 3.81 (s, 3H), 1.27 (s, 9H); 13C NMR (100 MHz, CDCl3) δ: 156.14, 143.29, 140.71, 134.83, 129.78, 125.24, 124.09, 114.82, 114.22, 110.88, 77.48, 77.16, 76.84, 55.70, 34.22, 31.52; IR (KBr) ν: 3396, 3036, 2961, 2904, 2867, 2833, 1606, 1511, 1463, 1441, 1391, 1362, 1317, 1296, 1280, 1247, 1180, 1115, 1105, 1037, 878, 842, 817, 772, 713, 664, 602 cm-1. HRMS (EI-TOF) C17H20NOBr 333.0728, found 333.0723.
1-溴-N-(4-甲氧苯基)萘-2-胺(4p):棕色液体, 产率38%. 1H NMR (400 MHz, CDCl3) δ: 8.08 (d, J=8.5 Hz, 1H), 7.69 (d, J=8.0 Hz, 1H), 7.61 (d, J=8.9 Hz, 1H), 7.52 (ddd, J=8.3, 6.9, 1.2 Hz, 1H), 7.33~7.27 (m, 1H), 7.23 (d, J=8.9 Hz, 1H), 7.19~7.14 (m, 2H), 6.96~6.88 (m, 2H), 6.36 (s, 1H), 3.83 (s, 3H); 13C NMR (100 MHz, CDCl3) δ: 156.68, 141.25, 134.43, 133.27, 129.13, 128.40, 128.26, 127.84, 125.36, 124.88, 123.27, 116.44, 114.92, 106.09, 77.48, 77.36, 77.16, 76.84, 55.71; IR (KBr) ν: 3390, 3053, 3001, 2954, 2928, 2833, 1622, 1603, 1558, 1505, 1463, 1428, 1409, 1352, 1282, 1243, 1180, 1150, 1106, 1035, 981, 938, 811, 761, 745, 661, 602 cm-1. HRMS (EI-TOF) calcd for C17H14NOBr 327.0259, found 327.0261.
2-甲基-4-(4-甲氧基苯基)苯并吗啉-3-酮(4ab)[15]:黄色固体, m.p. 143.8~144.4 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.18 (d, J=8.8 Hz, 2H), 7.07~6.94 (m, 4H), 6.90~6.81 (m, 1H), 6.44 (dd, J=8.1, 1.3 Hz, 1H), 4.80 (q, J=6.8 Hz, 1H), 3.86 (s, 3H), 1.64 (d, J=6.8 Hz, 4H); 13C NMR (100 MHz, CDCl3) δ: 167.04, 159.65, 144.24, 131.24, 129.89, 128.87, 124.03, 122.52, 117.36, 116.81, 115.33, 55.67, 16.52; IR (KBr) ν: 3434, 2961, 2937, 2840, 1687, 1609, 1591, 1512, 1497, 1462, 1445, 1371, 1327, 1297, 1270, 1253, 1169, 1130, 1108, 1049, 1029, 847, 815, 751, 684, 589, 576 cm-1. HRMS (EI-TOF) calcd for C16H15NO3 269.1052, found 269.1079.
-
-
[1]
(a) Yoo, E. J. ; Chang, S. Org. Lett. 2008, 10, 1163.
(b) Xing, Y. ; Sheng, G. ; Wang, J. ; Lu, P. ; Wang, Y. Org. Lett. 2014, 16, 1244.
(c) Senadi, G. C. ; Hu, W. P. ; Boominathan, S. S. K. ; Wang, J. J. Chem. Eur. J. 2015, 21, 998.
(d) Park, J. H. ; Kim, E. ; Chung, Y. K. Org. Lett. 2008, 10, 4719.
(e) Parisien, M. ; Valette, D. ; Fagnou, K. J. Org. Chem. 2005, 70, 7578.
(f) Numata, M. ; Yasuda, T. ; Adachi, C. Chem. Commun. 2015, 51, 9443.
(g) Liu, Z. ; Larock, R. C. Tetrahedron 2007, 63, 347.
(h) Li, X. ; Chianese, A. R. ; Vogel, T. ; Crabtree, R. H. ; Yang, Y. ; Zhang, X. ; Zeng, W. ; Huang, H. ; Liang, Y. Org. Lett. 2005, 7, 5437.
(i) Chen, Y. ; Cho, C. H. ; Shi, F. ; Larock, R. C. J. Org. Chem. 2009, 74, 6802.
(j) Andrew, T. L. ; Swager, T. M. J. Org. Chem. 2011, 76, 2976.
(k) Fang, X. ; Fang, L. ; Gou, S. Chin. J. Org. Chem. 2012, 32, 1217(in Chinese).
(房旭彬, 房雷, 苟少华, 有机化学, 2012, 32, 1217. )
(l) Xu, J. ; Wei, Z. ; Li, J. Chin. J. Org. Chem. 2012, 32, 1208(in Chinese).
(徐娟, 魏真, 李加荣, 有机化学, 2012, 32, 1208. ) -
[2]
(a) Guram, A. S.; Rennels, R. A.; Buchwald, S. L. Angew. Chem., Int. Ed. 1995, 34, 1348.
(b) Hartwig, J. F.; Kawatsura, M. S.; Hauck, I.; Shaughnessy, K. H.; Alcazar-Roman, L. M. J. Org. Chem. 1999, 64, 5575.
(c) Kuwano, R.; Utsunomiya, M.; Hartwig, J. F. J. Org. Chem. 2002, 67, 6479.
(d) Gajare, A. S.; Toyota, K.; Yoshifuji, M.; Ozawa, F. J. Org. Chem. 2004, 69, 6504.
(e) Monguchi, Y.; Kitamoto, K.; Ikawa, T.; Maegawa, T.; Sajiki, H. Adv. Synth. Catal. 2008, 350, 2767.
(f) Roiban, G. D.; Mehler, G.; Reetz, M. T. Eur. J. Org. Chem. 2014, 2014, 2070.
(g) Topchiy, M. A.; Dzhevakov, P. B.; Rubina, M. S.; Morozov, O. S.; Asachenko, A. F.; Nechaev, M. S. Eur. J. Org. Chem. 2016, 1908. -
[3]
(a) Rivera-Utrilla, J.; Bautista-Toledo, I.; Feffo-Garcia, M. A.; Moreno-Castilla, C. Carbon 2003, 41, 323.
(b) Lam, P. Y. S.; Clark, C. G.; Saubern, S.; Adams, J.; Winters, M. P.; Chan, D. M. T.; Combs, A. Tetrahedron Lett. 1998, 39, 2941.
(c) Antilla, J. C.; Buchwald, S. L. Org. Lett. 2001, 3, 2077.
(d) Lan, J. B.; Zhang, G. L.; Yu, X. Q.; You, J. S.; Chen, L.; Yan, M.; Xie, R. G. Synlett 2004, 1095.
(e) Gogoi, A.; Sarmah, G.; Dewan, A.; Bora, U. Tetrahedron Lett. 2014, 55, 31.
(f) Keesara, S. Tetrahedron Lett. 2015, 56, 6685.
(g) Yoo, W. J.; Tsukamoto, T.; Kobayashi, S. Angew. Chem., Int. Ed. 2015, 54, 6587.
(h) Roy, S.; Sarma, M. J.; Kashyap, B.; Phukan, P. Chem. Commun. 2016, 52, 1170. -
[4]
(a) Chan, D. M. T. ; Monaco, K. L. ; Wang, R. P. ; Winters, M. P. Tetrahedron Lett. 1998, 39, 2933.
(b) Garrett, C. E. ; Prasad, K. Adv. Synth. Catal. 2004, 346, 889.
(c) Lu, Q. ; Yi, H. ; Lei, A. Acta Chim. Sinica 2015, 73, 1245(in Chinese).
(陆庆全, 易红, 雷爱文, 化学学报, 2015, 73, 1245. ) -
[5]
Liu, Z. J.; Larock, R. C. Org. Lett. 2003, 5, 4673. doi: 10.1021/ol0358612
-
[6]
Carroll, M. A.; Wood, R. A. Tetrahedron 2007, 63, 11349. doi: 10.1016/j.tet.2007.08.076
-
[7]
Barros, M. T.; Dey, S. S.; Maycock, C. D.; Rodrigues, P. Chem. Commun. 2012, 48, 10901. doi: 10.1039/c2cc35801h
-
[8]
Yu, J. Z.; Wang, Y. T.; Zhang, P.; Wu, Z. J. Synlett 2013, 24, 1448. doi: 10.1055/s-00000083
-
[9]
Yu, J. Z.; Zhang, P. Z.; Wu, J.; Shang, Z. C. Tetrahedron Lett. 2013, 54, 3167. doi: 10.1016/j.tetlet.2013.04.028
-
[10]
Breugst, M.; Tokuyasu, T.; Mayr, H. J. Org. Chem. 2010, 75, 5250. doi: 10.1021/jo1009883
-
[11]
Newman, M. S. Acc. Chem. Res. 1972, 5, 354. doi: 10.1021/ar50058a006
-
[12]
Zuo, H.; Meng, L. J.; Ghate, M.; Hwang, K. H.; Cho, Y. K.; Chandrasekhar, S.; Reddy, C. R.; Shin, D. S. Tetrahedron Lett. 2008, 49, 3827. doi: 10.1016/j.tetlet.2008.03.120
-
[13]
Liu, Z.; Larock, R. C. Org. Lett. 2003, 5, 4673. doi: 10.1021/ol0358612
-
[14]
Barros, M. T.; Dey, S. S.; Maycock, C. D.; Rodrigues, P. Chem. Commun. 2012, 48, 10901. doi: 10.1039/c2cc35801h
-
[15]
Feng, E.; Huang, H.; Zhou, Y.; Ye, D.; Jiang, H.; Liu, H. J. Org. Chem. 2009, 74, 2846. doi: 10.1021/jo802818s
-
[1]
-
表 1 合成邻卤二芳胺4a的反应条件优化a
Table 1. Optimized condition for the synthesis of o-halo-diarylamines 4a

Entry Base Base/equiv. Time/h Conv./% Yieldb/% 4a 4ab 1 KOH 1.2 2 57 26 33 2 Cs2CO3 1.2 12 38 24 13 3 K2CO3 1.2 12 0 0 0 4 KOtBu 1.2 2 >99 0 46 5 KOH 2.4 4 — 30c — 6d KOH 1.2+1.2 2+2 — 33c — 7e KOH 1.2+1.2 1+1 — 28c — 8f KOH 1.2+1.2 2+2 — 66c — a Reagents and conditions: 3a(0. 2 mmol), base (0. 24 mmol) and DMSO (0.6 mL) were heated from room temperature to 130 ℃; bDetermined by 1H NMR of the crude reaction mixture; cIsolated yield; dThe base was added by two batches evenly each time at 130 ℃; eThe reaction mixture was heated by microwave and the base was added by two batches evenly each time; fThe second dose base was added after the mixture was cooled to 50 ℃. 表 2 非金属方法合成邻卤二芳胺4a
Table 2. Metal-free synthesis of o-halodiarylamines 4



a Reagents and conditions: (1) o-Halophenols 1(1. 0 mmol), 2-bromo-N-arylpropanamide 2(1.2 mmol), KOH (1.2 mmol), DMSO (3 mL), 50 ℃, 2 h; (2) KOH (1.2 mmol), 130 ℃, 2 h; (3) KOH (1.2 mmol) was added after the mixture was cooled to 50 ℃, then the mixture was heated to 130 ℃ again and stirred for 2 h; isolated yields were given. -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 3
- 文章访问数: 1650
- HTML全文浏览量: 232


 下载:
下载:

 下载:
下载:

