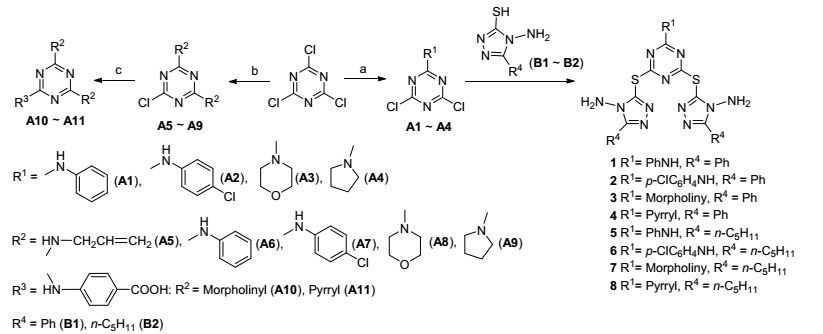
 图式 1
目标化合物1~8的合成方法
图式 1.
Synthetic methods of target compounds 1~8
图式 1
目标化合物1~8的合成方法
图式 1.
Synthetic methods of target compounds 1~8

Citation: Liu Yaning, Sun Xiaona, Gao Ran, Li Chuanyin, Wang Jing, Li Yizheng, Zhang Chenglu. Synthesis and Bioactivities of Multiheteocyclic Molecules Based on s-Triazine[J]. Chinese Journal of Organic Chemistry, 2017, 37(8): 2057-2065. doi: 10.6023/cjoc201701011

以均三嗪为核心的三类新型多杂环分子的合成与生物活性
-
关键词:
- 均三嗪
- / 1, 2, 4-三唑
- / 三唑并噻二唑
- / 1, 2, 4-三嗪
- / Cdc25B
English
Synthesis and Bioactivities of Multiheteocyclic Molecules Based on s-Triazine
-
Key words:
- s-triazine
- / 1, 2, 4-triazole
- / triazolo[3, 4-b]thiadiazole
- / 1, 2, 4-triazine
- / Cdc25B
-
癌症已成为导致全球疾病死亡的主要原因之一, 迫切需要研制新的和更安全的对肿瘤细胞具有更广谱毒性的抗癌剂, 杂环化合物因其结构的多样性和广泛的生物活性而倍受关注.
在各种人类癌症中, 细胞分裂周期25 (cell division cycle 25, 缩写Cdc25) 磷酸酯酶B (Cdc25B)是过度表达的, 因此Cdc25B成为抗癌治疗的一个重要靶标, 设计合成新型Cdc25B抑制剂无疑具有重要意义.将两个或更多杂环药效基团共筑在一个分子中, 可提供高度的结构多样性, 并已被证明其在治疗剂方面有广泛应用, 成为创制新型药物分子的最为有效的方法[1].
均三嗪衍生物是一类有显著药效的杂环化合物, 包括针对不同靶标的抗肿瘤活性[2~4]、抗菌[5]、抗炎[6]、抗疟活性[7, 8]等, 其关联深厚的医学应用, 吸引了众多合成工作者的兴趣.许多临床药物, 如用于治疗血癌的特里塔明(Tretamine), 用于肺癌、乳腺癌和卵巢癌等的六甲蜜胺(HMM)[9], 均含有均三嗪骨架结构, 因此针对均三嗪的结构修饰已成为获得活性优良抗癌药物分子最有效的途径[10~15]. 1, 2, 4-三唑也是一类极具吸引力的杂环化合物, 具有抗菌和抗病毒等生物活性[16~20].如临床药物特康唑、伊曲康唑、氟康唑、头孢唑啉、利巴韦林、三唑仑.阿普唑仑和乙替唑仑等均含有1, 2, 4-三唑核心结构, 因此含1, 2, 4-三唑的衍生物设计合成已成为近年药物研究与开发的重点领域之一.三唑并噻二唑衍生物也因其抗菌和抗肿瘤]等多样的生物活性而引起了广泛的关注[21~27], 尤其是最新发现具有该活性组块的分子具有抑制肿瘤扩散等药效[28~33].取代1, 2, 4-三嗪衍生物已成为许多药物、合成染料和除草剂中的关键组成, 在医药和农业领域具有重要的应用前景.
为了探究不同杂环对均三嗪修饰后, 新型分子对Cdc25B抑制活性的影响, 基于含1, 2, 4-三嗪、1, 3, 5-三嗪、1, 2, 4-三唑和三唑并噻二唑等活性组块分子的重要应用和本课题组前期研究的成果[34~36], 本文以均三嗪为核心, 分别借助活性硫醚键和亚氨键将上述不同结构的活性组块对接, 设计了三类迄今未见报道的21个新型多杂环分子, 期望通过评价其对Cdc25B的抑制活性, 发现并筛选抗癌药物先导物.研究中, 首先利用苯胺、对氯苯胺、吗啉和四氢吡咯修饰均三嗪得单取代中间体A1~A4, 进而分别与3-苯基-4-氨基-5-巯基-1, 2, 4-三唑和3-正戊基-4-氨基-5-巯基-1, 2, 4-三唑通过硫醚键对接, 得到目标分子1~8(如Scheme 1所示); 其次利用烯丙胺、苯胺、对氯苯胺、吗啉和四氢吡咯修饰均三嗪得双取代中间体A5~A9, 进而与3-氨基-5, 6-二苯基-1, 2, 4-三嗪(C)通过亚氨基对接, 得到目标分子9~13(如Scheme 2所示).为了评价三唑环上4-氨基对活性的影响, 选择目标分子7和8, 将分子中的4-氨基分别利用苯甲醛和对氯苯甲醛转化成席夫碱, 得到新目标分子14~17(如Scheme 3所示); 最后利用中间体A10与A11和中间体B1与B2, 通过缩合反应将均三嗪与三唑并噻二唑对接, 得到目标分子18~21(如Scheme 4所示).在目标分子设计中, 分别利用饱和脂肪含氮杂环、不饱和脂肪胺和芳香胺对均三嗪修饰, 以比较有无π电子以及三级胺和二级胺对活性的影响; 在对1, 2, 4-三唑环上氨基修饰时, 采用对氯苯基的原因一方面希望借助氯的吸电性研究电子迁移对活性的影响, 另一方面参考了药物分子中引入氯原子可增加活性的信息.
1 结果与讨论
1.1 目标分子的合成与表征
IR谱图中, 在3100和2900 cm-1左右出现苯环上C—H吸收峰, 1650和1500 cm-1左右出现C=N和C=C的吸收峰, 在1150和1100 cm-1左右出现C—O和C—N吸收峰; 1H NMR谱图中, δ 7.00~8.00处为芳环的质子信号, 吗啉环的质子信号在δ 2.8和3.7左右, NH上的质子信号在δ 10.0左右处; HRMS谱图中所有的化合物均出现了[M+1]+峰, 结果表明成功合成了目标分子.
1.2 对Cdc25B的抑制活性测试
目标分子初筛选择的浓度均为20 μg/mL, Na3VO4是酪氨酸磷酸酯酶的光谱抑制剂, 可作为Cdc25B抑制活性的阳性参照物.初筛后选择抑制率大于50%的分子进行复筛, 得出抑制活性剂量依赖关系, 即IC50值.抑制活性筛选实验方法参照考文献[37]的方法进行.本实验由国家新药中心协助完成, 测试结果见表 1所示.
TM IC50/(μg/mL) 1 NAb 2 2.63±0.16 3 NA 4 NA 5 2.77±0.11 6 1.11±0.05 7 NA 8 NA 9 0.97±0.07 10 1.13±0.42 11 2.15±0.70 12 NA 13 2.23±0.74 14 NA 15 3.99±0.80 16 NA 17 2.46±0.12 18 2.40±0.11 19 0.44±0.07 20 0.72±0.27 21 0.56±0.06 1.25±0.14 Positive control: Na3VO4 was for Cdc25B a Value tested at 5 μg/mL concentration; bnot active at 5 μg/mL concentration (inhibition rate≤50%). 2 结论
首次分别将1, 2, 4-三唑、三唑并噻二唑和1, 2, 4-三嗪等药效基团拼合在均三嗪结构中, 合成了21个新型多杂环目标分子, 其对Cdc25B抑制活性结果发现, 13个目标分子对Cdc25B具有良好的抑制活性, 其中6个分子的IC50值低于阳性参照物Na3VO4, 可作为潜在的Cdc25B抑制剂, 有望成为抗肿瘤药物先导物.
构效分析发现, 12个双1, 2, 4-三唑与均三嗪拼合的分子中(1~8, 14~17), 5个对Cdc25B表现出良好的抑制活性; 4个三唑并噻二唑与均三嗪拼合的目标分子(18~21), 均对Cdc25B表现出优秀的抑制活性; 5个1, 2, 4-三嗪与均三嗪拼合的分子(9~13)中, 4个对Cdc25B表现出良好的抑制活性, 表明将双1, 2, 4-三唑、三唑并噻二唑和1, 2, 4-三嗪与均三嗪拼合, 达到了预期分子设计的目的, 实现了活性叠加作用.进一步分析发现, 以四氢吡咯修饰的均三嗪衍生物活性优于以吗啉修饰的产物; 对氯苯亚氨基修饰的产物均比苯亚氨基修饰产物的活性高, 且相比吗啉和四氢吡咯修饰的分子活性高, 表明当均三嗪环上引入对氯苯亚氨基这一活性药效基团时, 可较好地提高分子的活性.对比含双1, 2, 4-三唑的目标分子(1~8, 14~17), 一方面三唑环3-位脂肪基产物比3-位芳香基产物活性提高, 可能是因为增大了目标分子的脂溶性所致, 另一方氨基修饰产物(14~17)与4-氨基未修饰产物(5~8)相比, 含有氨基和活性亚氨基产物5和6, 对比不含活性氨基和活性亚氨基产物14和15, 活性较好, 虽然通过席夫碱结构对电子密度产生了影响, 但分子中亚氨基N—H与生物大分子间可能发生的氢键, 使活性提高, 而且含对氯苯基的分子6和15均表现出较好的活性, 验证了预期分子设计中引入氯原子时可增加活性的判断, 均三嗪利用三级胺修饰时的分子7, 8和16活性不佳, 进一步证实了分子中亚氨基N—H与生物大分子间可能发生的氢键的判断, 由此说明氢键作用对分子生物活性的重要意义, 氨基未被修饰时可能因空间障碍对活性促进影响不大.三类不同结构的目标分子活性比较发现, 通过亚氨基拼合的分子比通过硫醚键拼合的分子活性更高, 可能的原因是前者更易与生物大分子产生氢键.
3 实验部分
3.1 仪器与试剂
AVANCE 500 MHz NMR核磁共振波谱仪; Waters X evo Q-TOF MS液相色谱-质谱联用仪; TENSOR 27傅立叶变换红外光谱仪; X-5型数字显微熔点测定仪(温度计未经校正); WFH-203B型三用紫外分析仪.所用的试剂均为市售的分析纯, 两个测试蛋白PTP1B、Cdc25B均由国家新药筛选中心实验室利用大肠杆菌表达并纯化得到的, 纯度在90%以上.化合物A1~A11, B3~B4, C由本课题组自行合成, 合成通法参照文献[38~40], B1根据文献[41]合成, 所得结果与文献值相符, B2参考文献[42]合成, 收率为65.7%, 熔点为105.1~106.4 ℃;
3.2 实验方法
3.2.1 目标分子1~8合成通法
在氮气氛围下, 将4 mmol 1, 2, 4-均三唑(B1, B2)分别加入到西朗特管中, 加入4 mL四氢呋喃(THF), 室温搅拌溶解后加入4 mmol KOH固体, 滴加2 mmol化合物(A1~A4)的2 mL THF溶液, 室温下搅拌0.5 h后回流6 h.过滤后得粗产品经乙醇重结晶后得到白色固体产品1~8.
6-苯氨基-2, 4-(5-硫醚基-4-氨基-3-苯基-1, 2, 4-三唑)-1, 3, 5-三嗪(1):白色固体, 收率84%. m.p. 249.1~250.2 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 10.38 (s, 1H, NH), 8.16~8.10 (m, 4H, Ph), 7.55~7.52 (m, 6H, Ph), 7.18 (d, J=8.00 Hz, 2H, Ph), 6.90 (t, J=7.65 Hz, 2H, Ph), 6.79 (t, J=7.30 Hz, 1H, Ph), 6.10 (s, 4H, NH2); 13C NMR (125 MHz, DMSO-d6) δ: 178.91 (triazine), 163.87 (triazine), 155.65 (triazole), 145.98 (triazole), 139.35 (Ph), 130.93 (Ph), 129.93 (Ph), 129.13 (Ph), 128.40 (Ph), 128.05 (Ph), 123.21 (Ph), 117.89 (Ph); IR (KBr) ν: 3323, 3050, 1618, 1580, 1513, 1292 cm-1; HRMS (positive-ESIMS) calcd for C25H21N12S2 (M+1)+ 553.1382, found 553.1375.
6-对氯苯氨基-2, 4-(5-硫醚基-4-氨基-3-苯基-1, 2, 4-三唑)-1, 3, 5-三嗪(2):白色固体, 收率71%. m.p. 266.5~267.8 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 10.83 (s, 1H, NH), 8.10~8.02 (m, 4H, Ph), 7.52~7.48 (m, 6H, Ph), 7.12 (d, J=8.85 Hz, 2H, Ph), 6.89 (d, J=8.90 Hz, 2H, Ph), 6.13 (s, 2H, NH2), 6.07 (s, 2H, NH2); 13C NMR (125 MHz, DMSO-d6)δ: 178.91 (triazine), 163.87 (triazine), 155.65 (triazole), 145.98 (triazole), 137.46 (p-chloroa-niline), 130.95 (Ph), 129.93 (Ph), 129.25 (p-chloroaniline), 128.41 (Ph), 128.36 (p-chloroaniline), 128.08 (Ph), 121.81 (p-chloroaniline); IR (KBr) ν: 3330, 3037, 1561, 1620, 1582, 1515, 1292 cm-1; HRMS (positive-ESIMS) calcd for C25H20ClN12S2 (M+1)+ 587.0993, found 587.0986.
6-吗啉-2, 4-(5-巯基-4-氨基-3-苯基-1, 2, 4-三唑)-1, 3, 5-三嗪(3):白色固体, 收率81%. m.p. 253.7~254.5 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 8.04~8.01 (m, 4H, Ph), 7.56~7.51 (m, 6H, Ph), 6.05 (s, 4H, NH2), 3.45 (t, J=4.70 Hz, 4H, morpholine), 3.36 (t, J=4.70 Hz, 4H, morpholine); 13C NMR (125 MHz, DMSO-d6) δ: 176.68 (triazine), 160.78 (triazine), 154.80 (triazole), 145.53 (triazole), 130.95 (Ph), 129.93 (Ph), 128.40 (Ph), 128.01 (Ph), 65.16 (morpholine), 43.25 (morpholine); IR (KBr) ν: 3298, 3258, 3103, 2866, 1615, 1583, 1511, 1292, 1252 cm-1; HRMS (positive-ESIMS) calcd for C23H23N12-OS2 (M+1)+ 547.1491, found 547.1481.
6-吡咯-2, 4-(5-巯基-4-氨基-3-苯基-1, 2, 4-三唑)-1, 3, 5-三嗪(4):白色固体, 收率82%. m.p. 251.8~252.4 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 8.06~8.04 (m, 4H, Ph), 7.54~7.50 (m, 6H, Ph), 6.04 (s, 4H, NH2), 3.12 (t, J=6.50 Hz, 4H, pyrrolidine), 1.86~1.81 (m, 4H, pyrrolidine); 13C NMR (125 MHz, DMSO-d6)δ: 176.69 (triazine), 160.78 (triazine), 154.81 (triazole), 145.53 (triazole), 130.95 (Ph), 129.93 (Ph), 128.40 (Ph), 128.01 (Ph), 53.61 (pyrrolidine), 24.16 (pyrrolidine); IR (KBr) ν: 3230, 3258, 3033, 2867, 1612, 1583, 1512, 1292 cm-1; HRMS (positive-ESIMS) calcd for C23H23N12S2 (M+1)+531.1541, found 531.1532.
6-苯氨基-2, 4-(5-硫醚基-4-氨基-3-正戊基-1, 2, 4-三唑)-1, 3, 5-三嗪(5):白色固体, 收率78%. m.p. 239.1~240.3 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 10.36 (s, 1H, NH), 7.15 (d, J=8.00 Hz, 2H, Ph), 6.79 (t, J=7.30 Hz, 1H, Ph), 6.12 (s, 2H, NH2), 2.88 (t, J=7.20 Hz, 2H, CH2), 1.80 (t, J=7.20 Hz, 2H, CH2), 1.36 (t, J=7.00 Hz, 4H, CH2), 0.90 (t, J=7.00 Hz, 3H, CH3); 13C NMR (125 MHz, DMSO-d6) δ: 178.90 (triazine), 163.88 (triazine), 155.66 (triazole), 145.97 (triazole), 128.40 (Ph), 128.00 (Ph), 123.20 (Ph), 117.88 (Ph), 31.22 (alkyl), 25.81 (alkyl), 25.00 (alkyl), 22.11 (alkyl), 13.88 (alkyl); IR (KBr) ν: 3320, 3050, 1616, 1580, 1518, 2940, 2856, 745, 1296 cm-1; HRMS (positive-ESIMS) calcd for C23H33N12S2 (M+1)+ 541.2302, found 541.2311.
6-对氯苯氨基-2, 4-(5-硫醚基-4-氨基-3-正戊基-1, 2, 4-三唑)-1, 3, 5-三嗪(6):白色固体, 收率76%. m.p. 235.5~236.3 ℃; 1H NMR (500 MHZ, DMSO-d6) δ: 10.83 (s, 1H, NH), 7.12 (d, J=8.85 Hz, 2H, Ph), 6.12 (s, 2H, NH2), 2.84 (t, J=7.20 Hz, 2H, CH2), 1.80 (t, J=7.20 Hz, 2H, CH2), 1.38 (t, J=7.00 Hz, 4H, CH2), 0.88 (t, J=7.00 Hz, 3H, CH3); 13C NMR (125 MHz, DMSO-d6)δ: 178.99 (triazine), 163.85 (triazine), 155.68 (triazole), 145.98 (triazole), 137.45 (Ph), 129.26 (Ph), 128.36 (Ph), 121.80 (Ph), 31.22 (alkyl), 25.71 (alkyl), 25.03 (alkyl), 22.15 (alkyl), 13.85 (alkyl); IR (KBr) ν: 3330, 3030, 1561, 1620, 1588, 1517, 2949, 2850, 745, 1290 cm-1; HRMS (positive-ESIMS) calcd for C23H32ClN12S2(M+1)+ 575.1920, found 575.1925.
6-吗啉-2, 4-(5-硫醚基-4-氨基-3-正戊基-1, 2, 4-三唑)-1, 3, 5-三嗪(7):白色固体, 收率79%. m.p. 241.7~243.1 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 6.06 (s, 2H, NH2), 3.48 (t, J=4.70 Hz, 2H, morpholine), 3.36 (t, J=4.70 Hz, 2H, morpholine), 2.89 (t, J=7.20 Hz, 2H, CH2), 1.80 (t, J=7.20 Hz, 2H, CH2), 1.36 (t, J=7.00 Hz, 4H, CH2), 0.91 (t, J=7.00 Hz, 3H, CH3); 13C NMR (125 MHz, DMSO-d6) δ: 176.65 (triazine), 160.76 (triazine), 154.80 (triazole), 145.51 (triazole), 65.18 (morpholine), 43.26 (morpholine), 31.20 (alkyl), 25.70 (alkyl), 25.05 (alkyl), 22.12 (alkyl), 13.89 (alkyl); IR (KBr) ν: 3290, 3255, 3100, 2860, 2944, 2858, 1616, 1583, 1515, 1298, 1254, 745 cm-1; HRMS (positive-ESIMS) calcd for C21H35N12OS2 (M+1)+ 535.2430, found 535.2420.
6-吡咯-2, 4-(5-巯基-4-氨基-3-正戊基-1, 2, 4-三唑)-1, 3, 5-三嗪(8):白色固体, 收率80%. m.p. 255.5~256.9 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 6.19 (s, 2H, NH2), 3.22 (d, J=4.90 Hz, 2H, pyrrolidine), 2.80 (t, J=7.20 Hz, 2H, CH2), 1.88 (t, J=7.20 Hz, 2H, CH2), 1.38~1.35 (m, 2H, CH2), 1.33 (t, J=7.00 Hz, 4H, CH2), 0.84 (t, J=7.00 Hz, 3H, CH3); 13C NMR (125 MHz, DMSO-d6)δ: 176.66 (triazine), 160.77 (triazine), 154.83 (triazole), 145.52 (triazole), 53.62 (pyrrolidine), 31.20 (alkyl), 25.71 (alkyl), 25.04 (alkyl), 24.15 (pyrrolidine), 22.11 (alkyl), 13.87 (alkyl); IR (KBr) ν: 3233, 3250, 2949, 2850, 1699, 1385, 1279, 876, 746 cm-1; HRMS (positive-ESIMS) calcd for C21H35N12S2 (M+1)+ 519.2455, found 519.2463.
3.2.2 目标化合物9~13合成通法
冰浴下将0.155 g (0.001 mol) 3-氨基-5, 6-二苯基-1, 2, 4-三嗪(C)和10 mL THF加入三口瓶中, 搅拌溶解后, 加入0.08 g的NaOH, 0 ℃下搅拌1 h, 将均三嗪衍生物A5~A9的THF溶液分别逐滴滴加到反应体系中, 搅拌30 min, 室温继续反应1 h后回流反应, 薄层色谱(TLC)监控反应, 待反应完毕后, 冷却至室温, 过滤, 浓缩滤液, 用柱层析分离(V石油醚:V乙酸乙酯=3:1), 减压蒸除溶剂, 得到产物9~13, 收率53.2%~73.5%.
N, N-(5, 6-二苯基-1, 2, 4-三嗪-3-基)-(2, 4-二烯丙胺基-1, 3, 5-三嗪-6-基)胺(9):黄色固体, 收率59.7%. m.p. 176.5~177.2 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 8.92 (s, 1H, NH), 8.32 (s, 2H, NH), 7.51 (d, J=7.25 Hz, 2H, PhH), 7.46 (d, J=7.45 Hz, 2H, PhH), 7.40~7.37 (m, 2H, PhH), 7.34~7.25 (m, 4H, PhH), 5.86~5.84 (m, 2H, CH2CH=CH2), 5.13 (d, J=6.25 Hz, 4H, CH2CH=CH2), 3.92 (d, J=6.75 Hz, 4H, CH2CH=CH2); 13C NMR (125 MHz, DMSO-d6) δ: 165.46 (triazine, C×2), 163.93 (triazine), 161.96 (triazine), 157.27 (triazine), 151.13 (triazine), 135.96 (Ph), 135.85 (CH2-CH=CH2, C×2), 135.73 (Ph), 130.82 (Ph), 129.87 (Ph), 129.37 (Ph, C×2), 129.07 (Ph, C×2), 128.60 (Ph, C×2), 128.27 (Ph, C×2), 118.46 (CH2-CH=CH2, 2×C), 48.25 (CH2-CH=CH2, 2×C); IR (KBr) ν: 3401.4, 3345.5, 3069.3, 2916.8, 1631.4, 1572.8, 1163.3, 731.2 cm-1; HRMS (positive-ESIMS) calcd for C24H24N9 (M+1)+ 438.2057, found 438.2066.
N, N-(5, 6-二苯基-1, 2, 4-三嗪-3-基)-(2, 4-二苯胺基-1, 3, 5-三嗪-6-基)胺(10):浅黄色固体, 收率72.7%. m.p. 257.3~258.1 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 8.63 (s, 3H, NH), 8.09 (t, J=7.35 Hz, 4H, PhH), 7.62~7.58 (m, 6H, PhH), 7.47 (d, J=7.25 Hz, 2H, PhH), 7.41 (t, J=7.75 Hz, 2H, PhH), 7.35~7.29 (m, 6H, PhH); 13C NMR (125 MHz, DMSO-d6) δ: 168.36 (triazine, C×2), 163.63 (triazine), 161.27 (triazine), 157.24 (triazine), 151.63 (triazine), 139.28 (Ph', 2×C), 136.07 (Ph), 135.83 (Ph), 130.86 (Ph), 130.24 (Ph', C×4), 130.15 (Ph), 129.75 (Ph, C×2), 129.31 (Ph, C×2), 128.77 (Ph, C×2), 128.45 (Ph, C×2), 122.57 (Ph', C×2), 118.76 (Ph', C×4); IR (KBr) ν: 3047.3, 2938.6, 1634.3, 1590.4, 1072.5, 732.6 cm-1; HRMS (positive-ESIMS) calcd for C30H24N9 (M+1)+ 510.2089, found 510.2086.
N, N-(5, 6-二苯基-1, 2, 4-三嗪-3-基)-{2, 4-二(4-氯苯胺基)-1, 3, 5-三嗪-6-基}胺(11):浅黄色固体, 收率73.2%. m.p. 285.3~285.9 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 9.04 (s, 3H, NH), 8.49 (d, J=6.75 Hz, 4H, PhH), 7.65 (t, J=6.5 Hz, 2H, PhH), 7.59~7.58 (m, 2H, PhH), 7.44~7.41 (m, 2H, PhH), 7.38~7.33 (m, 4H, PhH), 6.80 (dd, J=1.9 Hz, J=6.8 Hz, 4H, PhH); 13C NMR (125 MHz, DMSO-d6) δ: 168.35 (triazine, C×2), 163.77 (triazine), 161.26 (triazine), 157.17 (triazine), 151.26 (triazine), 138.25 (Ph', C×2), 136.33 (Ph), 136.14 (Ph), 130.96 (Ph), 130.07 (Ph), 129.83 (Ph, C×2), 129.68 (Ph', C×4), 129.54 (Ph, C×2), 128.82 (Ph, C×2), 128.40 (Ph, C×2), 127.26 (Ph', C×2), 123.65 (Ph', C×4); IR (KBr) ν: 3089.8, 2906.5, 1662.4, 1582.8, 1172.1, 728.3 cm-1; HRMS (positive-ESIMS) calcd for C30H22Cl2N9(M+1)+ 578.1271, found 578.1277.
N, N-(5, 6-二苯基-1, 2, 4-三嗪-3-基)-(2, 4-二吗啉基-1, 3, 5-三嗪-6-基)胺(12):黄色晶状固体, 收率70.2%. m.p. 241.3~243.2 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 8.74 (s, 1H, NH), 7.42~7.36 (m, 5H, PhH), 7.33~7.29 (m, 5H, PhH), 3.67 (t, J=4.15 Hz, 8H, CH2NCH2), 3.61 (t, J=4.05 Hz, 8H, CH2OCH2); 13C NMR (125 MHz, CDCl3)δ: 165.42 (triazine, C×2), 163.49 (triazine), 157.74 (triazine, 156.20 (triazine), 152.54 (triazine), 135.91 (Ph), 135.72 (Ph), 130.68 (Ph), 129.85 (Ph), 129.33 (Ph, C×2), 129.05 (Ph, C×2), 128.56 (Ph, C×2), 128.25 (Ph, C×2), 66.82 (morpholine), 43.74 (morpholine); IR (KBr) ν: 3330.3, 3036.6, 2928.4, 1624.8, 1596.4, 1030.2 cm-1; HRMS (positive-ESIMS) calcd for C26H28N9O2(M+1)+ 498.2301, found 498.2298.
N, N-(5, 6-二苯基-1, 2, 4-三嗪-3-基)-(2, 4-二吡咯烷-1, 3, 5-三嗪-6-基)胺(13):黄色固体, 收率73.2%. m.p. 248.3~249.6 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 8.98 (s, 1H, NH), 7.54~7.50 (m, 2H, PhH), 7.38 (d, J=7.2 Hz, 2H, PhH), 7.30 (d, J=7.25 Hz, 2H, PhH), 6.98~6.92 (m, 4H, PhH), 3.67 (t, J=4.15 Hz, 8H, pyrrolidine), 1.85~1.80 (m, 8H, pyrrolidine); 13C NMR (125 MHz, CDCl3) δ: 165.41 (triazine, C×2), 163.46 (triazine), 157.77 (triazine), 156.22 (triazine), 152.53 (triazine), 135.95 (Ph), 135.75 (Ph), 130.66 (Ph), 129.88 (Ph), 129.36 (Ph, C×2), 129.08 (Ph, C×2), 128.58 (Ph, C×2), 128.26 (Ph, C×2), 54.15 (pyrrolidine), 25.22 (pyrrolidine); IR (KBr) ν: 3376, 3047.3, 2938, 1634, 1598, 1388, 1270, 733 cm-1; HRMS (positive-ESIMS) calcd for C26H28N9(M+1)+ 466.2371, found 466.2379.
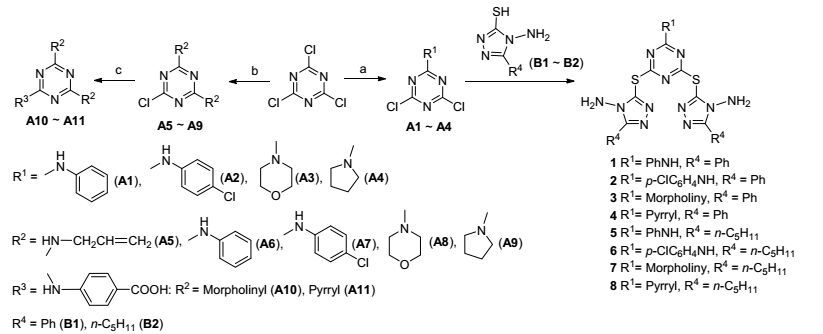
3.2.3 目标化合物14~17合成通法
将Schiff碱(B3或B4, 5×10-4 mol)溶于10 mL的THF中(温度为66 ℃), 加入0.056 g KOH (1×10-3 mol), 搅拌, 溶液由澄清变成黄色浑浊, 加入三嗪单取代物(A8或A9, 2.5×10-4 mol), 反应24 h, 静止, 冷却至室温, 将固体抽滤, 固体用乙醚洗涤2次后用石油醚:乙酸乙酯(V:V=3:1) 进行柱层析的目标产物.
二-N-苯亚基-3-((4-((4-((苯亚基)氨基-5-苯基-4H-1, 2, 4-三唑-3-基)硫醚)-6-吗啡啉-1, 3, 5-三嗪-2-基)硫醚)-5-苯基-4H-1, 2, 4-三唑-4-胺(14):浅黄色固体, 收率70.4%. m.p. 234.2~235.8 ℃; 1H NMR (500 MHz, CDCl3) δ: 8.24 (s, 1H, CH=N), 8.00 (d, J=8.30 Hz, 2H, PhH), 6.96 (d, J=8.30 Hz, 3H, PhH), 3.73 (t, J=5.00 Hz, 2H, CH2OCH2), 3.55 (t, J=4.75 Hz, 2H, CH2NCH2), 2.85 (t, J=7.20 Hz, 2H, CH2), 1.81 (t, J=7.20 Hz, 2H, CH2), 1.36 (t, J=7.00 Hz, 4H, CH2), 0.90 (t, J=7.00 Hz, 3H, CH3); 13C NMR (125 MHz, CDCl3) δ: 176.74 (triazine), 163.52 (triazole), 162.50 (triazole), 160.70 (triazine), 150.84(N=CH), 130.94 (Ph), 129.92 (Ph), 128.38 (Ph), 128.00 (Ph), 65.18 (morpholine), 43.23 (morpholine), 31.20 (alkyl), 25.80 (alkyl), 25.01 (alkyl), 22.14 (alkyl), 13.85 (alkyl); IR (KBr) ν: 3030, 2940, 2850, 1690, 1600, 1510, 1450, 1375, 1290, 1250, 745 cm-1; HRMS (positive-ESIMS) calcd for C35H43N12OS2 (M+1)+ 711.3038, found 711.3046.
二-N-(4-氯苯亚基)-3-((4-((4-((4-氯苯亚基)氨基-5-苯基-4H-1, 2, 4-三唑-3-基)硫醚)-6-吗啡啉-1, 3, 5-三嗪-2-基)硫醚)-5-苯基-4H-1, 2, 4-三唑-4-胺(15):黄色固体, 收率71.2%. m.p. 187.8~188.9 ℃; 1H NMR (500 Hz, CDCl3) δ: 8.30 (s, 1H, CH=N), 7.81~7.77 (m, 2H, PhH), 7.45~7.42 (m, 2H, PhH), 3.73 (t, J=5.00 Hz, 2H, CH2OCH2), 3.54 (t, J=4.75 Hz, 2H, CH2NCH2), 2.85 (t, J=7.20 Hz, 2H, CH2), 1.81 (t, J=7.20 Hz, 2H, CH2), 1.36 (t, J=7.00 Hz, 4H, CH2), 0.90 (t, J=7.00 Hz, 3H, CH3); 13C NMR (125 MHz, CDCl3) δ: 176.64 (triazine), 163.06 (triazole), 162.60 (triazole), 161.40 (Ph), 160.78 (triazine), 150.82(N=CH), 130.74 (Ph), 129.04 (Ph), 116.28 (Ph), 65.10 (morpholine), 43.20 (morpholine), 31.24 (alkyl), 25.84 (alkyl), 25.00 (alkyl), 22.14 (alkyl), 13.84 (alkyl); IR (KBr) ν: 3030, 2925, 2850, 1686, 1600, 1500, 1450, 1380, 1290, 1250, 750 cm-1; HRMS (positive-ESIMS) calcd for C35H41Cl2N12OS2(M+1)+ 779.2259, found 779.2267.
二-N-(苯亚基)-3-((4-((4-((苯亚基)氨基-5-苯基-4H-1, 2, 4-三唑-3-基)硫醚)-6-吡咯烷-1, 3, 5-三嗪-2-基)硫醚)-5-苯基-4H-1, 2, 4-三唑-4-胺(16):浅黄色固体, 收率68.8%. m.p. 178.4~179.6 ℃; 1H NMR (500 Hz, CDCl3) δ: 8.24 (s, 1H, CH=N), 7.96 (d, J=8.75 Hz, 2H, PhH), 7.11 (d, J=8.75 Hz, 3H, PhH), 3.73 (t, J=4.95 Hz, 2H, CH2N), 3.55 (t, J=5.90 Hz, 2H, CH2), 2.85 (t, J=7.50 Hz, 2H, CH2), 1.81 (t, J=7.45 Hz, 2H, CH2), 1.38~1.32 (m, 4H, CH2), 0.90(q, J=7.50 Hz, 3H, CH3); 13C NMR (125 MHz, CDCl3) δ: 176.74 (triazine), 163.52 (triazole), 162.50 (triazole), 160.70 (triazine), 150.84 (N=CH), 130.94 (Ph), 129.92 (Ph), 128.38 (Ph), 128.00 (Ph), 54.16 (pyrrolidine), 31.20 (alkyl), 25.80 (alkyl), 25.24 (pyrrolidine), 25.01 (alkyl), 22.14 (alkyl), 13.85 (alkyl); IR (KBr) ν: 3030, 2940, 2850, 1690, 1600, 1510, 1450, 1375, 1270, 876, 745 cm-1; HRMS (positive-ESIMS) calcd for C35H43-N12S2 (M+1)+ 695.3088, found 695.3097.
二-N-(4-氯苯亚基)-3-((4-((4-((4-氯苯亚基)氨基-5-苯基-4H-1, 2, 4-三唑-3-基)硫醚)-6-吡咯烷-1, 3, 5-三嗪-2-基)硫醚)-5-苯基-4H-1, 2, 4-三唑-4-胺(17):淡黄色固体, 收率77.8%. m.p. 205.7~206.6 ℃; 1H NMR (500 Hz, CDCl3) δ: 8.24 (s, 1H, CH=N), 7.88~7.85 (m, 2H, PhH), 7.26~7.15 (m, 2H, PhH), 3.73 (t, J=4.95 Hz, 2H, CH2N), 3.55 (t, J=4.95 Hz, 2H, CH2), 2.85 (t, J=7.50 Hz, 2H, CH2), 1.81 (t, J=7.45 Hz, 2H, CH2), 1.38~1.32 (m, 4H, CH2), 0.89 (q, J=7.50 Hz, 3H, CH3); 13C NMR (125 MHz, CDCl3) δ: 176.64 (triazine), 163.06 (triazole), 162.60 (triazole), 161.40 (Ph), 160.78 (triazine), 150.82(N=CH), 130.74 (Ph), 129.04 (Ph), 116.28 (Ph), 54.16 (pyrrolidine), 31.24 (alkyl), 25.84 (alkyl), 25.24 (pyrrolidine), 25.00 (alkyl), 22.14 (alkyl), 13.84 (alkyl); IR (KBr) ν: 3033, 2950, 2850, 1690, 1610, 1520, 1450, 1384, 1295, 830, 740 cm-1; HRMS (positive-ESIMS) calcd for C35H41Cl2N12S2 (M+ 1)+ 763.2311, found 763.2317.
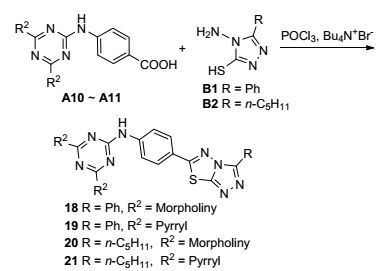
3.2.4 目标化合物18~21合成通法
在氩气氛围下, 将0.5 mmol化合物(B1或B2)和0.2 mmol四丁基溴化铵加入到西朗克管中, 向其中加入10 mL POCl3, 升温至90 ℃, 分批加入0.6 mmol (A10或A11), 加毕升温至100 ℃, 反应6 h.冷却, 减压蒸出POCl3, 黄色油状物加入到100 mL冰水中, 剧烈搅拌, 用K2CO3和KOH的25%的混合溶液调pH至6~7, 静置过夜, 过滤出沉淀物, 冷水洗涤, 乙醇和DMF混合溶液重结晶, 干燥得到黄色固体产物18~21.
4, 6-双吗啉-2-(3-苯基-(1, 2, 4-三唑并噻二唑)-1, 3, 5-三嗪(18):黄色固体, 收率73%. m.p. 230.6~231.3 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 9.65 (s, 1H, NH), 8.34 (d, J=7.30 Hz, 2H, Ph), 7.85 (d, J=8.95 Hz, 2H, Ph), 7.65~7.57 (m, 3H, Ph), 6.87 (d, J=8.45 Hz, 2H, Ph), 3.73 (t, J=4.70 Hz, 8H, morpholine), 3.65 (t, J=4.70 Hz, 8H, morpholine); 13C NMR (125 MHz, DMSO-d6) δ: 172.61 (triazine), 165.28 (triazine), 162.72 (triazole), 151.12 (triazole), 143.88 (thiadiazole), 138.93 (Ph), 131.82 (Ph), 130.91 (Ph), 129.43 (Ph), 128.95 (Ph), 127.31 (Ph), 123.55 (Ph), 116.82 (Ph), 66.38 (morpholine), 44.71 (morpholine); IR (KBr) ν: 3422, 3049, 2915, 1613, 1581, 1523, 1381, 1292, 1149 cm-1; HRMS (positive-ESIMS) calcd for C26H27N10O2S (M+1)+ 543.2034, found 543.2035.
4, 6-双四氢吡咯-2-(3-苯基-(1, 2, 4-三唑并噻二唑)-1, 3, 5-三嗪(19):黄色固体, 收率71%. m.p. 235.0~235.9 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 9.65 (s, 1H, NH), 8.35 (d, J=7.30 Hz, 2H, Ph), 7.84 (d, J=8.90 Hz, 2H, Ph), 7.65~7.57 (m, 3H, Ph), 6.87 (d, J=8.85 Hz, 2H, Ph), 3.59 (t, J=6.85 Hz, 8H, pyrrolidine), 1.98~1.96 (m, 8H, pyrrolidine); 13C NMR (125 MHz, DMSO-d6)δ: 172.61 (triazine), 165.28 (triazine), 162.72 (triazole), 151.12 (triazole), 143.88 (thiadiazole), 138.93 (Ph), 131.82 (Ph), 130.91 (Ph), 129.43 (Ph), 128.95 (Ph), 127.31 (Ph), 123.55 (Ph), 116.82 (Ph), 55.47 (pyrrolidine), 25.51 (pyrrolidine); IR (KBr) ν: 3423, 3061, 2914, 1613, 1581, 1515, 1386, 1293 cm-1; HRMS (positive-ESIMS) calcd for C26H27N10S (M+1)+ 511.2045, found 511.2052.
4, 6-双吗啉-2-(3-正戊基-(1, 2, 4-三唑并噻二唑)-1, 3, 5-三嗪(20):黄色固体, 收率80%. m.p. 188.9~189.4 ℃; 1H NMR (500 MHz, DMSO-d6) δ: 9.62 (s, 1H, NH), 7.93 (d, J=7.10 Hz, 2H, Ph), 6.86 (d, J=7.20 Hz, 2H, Ph), 3.76 (t, J=4.70 Hz, 8H, morpholine), 3.67 (t, J=4.50 Hz, 8H, morpholine), 2.79 (t, J=7.50 Hz, 2H, CH2), 1.63~1.59 (m, 2H, CH2), 1.39~1.33 (m, 4H, CH2), 0.90 (t, J=7.50 Hz, 3H, CH3); 13C NMR (125 MHz, DMSO-d6) δ: 172.62 (triazine), 165.27 (triazine), 162.73 (triazole), 160.52 (triazole), 143.86(thiadiazole), 138.91 (Ph), 129.44 (Ph), 123.55 (Ph), 116.82 (Ph), 66.38 (morpholine), 44.71 (morpholine), 31.48 (alkyl), 25.80 (alkyl), 24.99 (alkyl), 22.16 (alkyl), 14.17 (alkyl); IR (KBr) ν: 3429, 3033, 2931, 1622, 1586, 1513 1380, 1294, 1153 cm-1; HRMS (positive-ESIMS) calcd for C25H33N10S (M+1)+ 537.2433, found 537.2430.
4, 6-双四氢吡咯-2-(3-正戊基-(1, 2, 4-三唑并噻二唑)-1, 3, 5-三嗪(21):黄色固体, 收率81%. m.p. 199.8~200.2 ℃; 1H NMR (500 MHz, CDCl3) δ: 9.90 (s, 1H, NH), 7.99 (d, J=8.30 Hz, 2H, Ph), 6.68 (d, J=8.60 Hz, 2H, Ph), 3.69 (t, J=6.45 Hz, 8H, pyrrolidine), 2.89 (t, J=7.50 Hz, 2H, CH2), 1.95~1.91 (m, 8H, pyrrolidine), 1.63~1.59 (m, 2H, CH2), 1.40~1.35 (m, 4H, CH2), 0.90 (t, J=7.50 Hz, 3H, CH3); 13C NMR (125 MHz, CDCl3) δ: 172.36 (triazine), 165.09 (triazine), 162.48 (triazole), 159.84 (triazole), 143.01 (thiadiazole), 138.63 (Ph), 129.43 (Ph), 123.25 (Ph), 116.53 (Ph), 54.17 (pyrrolidine), 31.18 (alkyl), 25.56 (pyrrolidine), 22.85 (alkyl), 22.03 (alkyl), 21.25 (alkyl), 14.01 (alkyl); IR (KBr) ν: 3404, 3193, 2961, 1618, 1580, 1523, 1381, 1274 cm-1; HRMS (positive-ESIMS) calcd for C25H33N10S (M+1)+ 505.2510, found 505.2518.
辅助材料(Supporting Information) 化合物的核磁共振氢谱和核磁共振碳谱.这些材料可以免费从本刊网站(http://sioc-journal.cn/)上下载.
-
-
[1]
Sharma, N.; Mohanakrishnan, D.; Shard, A.; Sharma, A.; Saima, A. K.; Sinha, D. Sahal J. Med. Chem. 2012, 55, 297.
-
[2]
Saczewski, F.; Bułakowska, A. Eur. J. Med. Chem. 2006, 41, 611. doi: 10.1016/j.ejmech.2005.12.012
-
[3]
Zheng, M.; Xu, C.; Ma, J.; Sun, Y.; Du, F.; Liu, H.; Lin, L.; Li, C.; Ding, J.; Chen, K.; Jiang, H. Bioorg. Med. Chem. 2007, 15, 1815. doi: 10.1016/j.bmc.2006.11.028
-
[4]
Liu, K.; Xia, B.; Ma, W.; Zheng, B.; Zhang, X.; Fan, B. QSAR Comb. Sci. 2008, 27, 425. doi: 10.1002/(ISSN)1611-0218
-
[5]
El-Faham, A.; Soliman, S. M.; Ghabbour, H. A.; Elnakady, Y. A.; Mohaya, T. A.; Siddiqui, M. R. H. J. Mol. Struct. 2016, 1125, 121. doi: 10.1016/j.molstruc.2016.06.061
-
[6]
Bennett, G. B.; Mason, R. B.; Alden, L. J.; RoachJr, J. B. J. Med. Chem. 1978, 21, 623. doi: 10.1021/jm00205a006
-
[7]
Ojha, H.; Gahlot, P.; Tiwari, A. K.; Pathak, M.; Kakkar, R. Chem. Biol. Drug Des. 2011, 77, 57. doi: 10.1111/j.1747-0285.2010.01045.x
-
[8]
Agarwal, A.; Srivastava, K.; Puri, S. K.; Chauhan, P. M. S. Bioorg. Med. Chem. Lett. 2005, 15(3), 531. doi: 10.1016/j.bmcl.2004.11.052
-
[9]
Kapil, A.; Anshu, D. Bioorg. Med. Chem. 2007, 17, 3298. doi: 10.1016/j.bmcl.2007.04.007
-
[10]
Mallon, R.; Feldberg, L. R.; Lucas, J.; Chaudhary, I.; Dehnhardt, C.; Santos, E. D.; Chen, Z.; Dos Santos, O.; Ayral-Kaloustian, S.; Venkatesan, A.; Hollander, I. Clin. Cancer Res. 2011, 17, 3193. doi: 10.1158/1078-0432.CCR-10-1694
-
[11]
Burger, M. T.; Pecchi, S.; Wagman, A.; Ni, Z. J.; Knapp, M.; Hendrickson, T.; Atallah, G.; Pfister, K.; Zhang, Y.; Bartulis, S.; Frazier, K.; Ng, S.; Smith, A.; Verhagen, J.; Haznedar, J.; Huh, K.; Iwanowicz, E.; Xin, X.; Menezes, D.; Merritt, H.; Lee, I.; Wiesmann, M.; Kaufman, S.; Crawford, K.; Chin, M.; Bussiere, D.; Shoemaker, K.; Zaror, I.; Maira, S. M.; Voliva, C. F. ACS. Med. Chem. Lett. 2011, 2, 774. doi: 10.1021/ml200156t
-
[12]
Maira, S. M.; Pecchi, S.; Huang, A.; Burger, M.; Knapp, M.; Sterker, D.; Schnell, C.; Guthy, D.; Nagel, T.; Wiesmann, M.; Brachmann, S.; Fritsch, C.; Dorsch, M.; Chene, P.; Shoemaker, K.; Pover, A. D.; Menezes, D.; Martiny-Baron, G.; Fabbro, D.; Wilson, C. J.; Schlegel, R.; Hofmann, F.; Garcıa-Echeverrıa, C.; Sellers, W. R.; Voliva, C. F. Mol. Cancer. Ther. 2012, 11, 317. doi: 10.1158/1535-7163.MCT-11-0474
-
[13]
Sarıpınar, E.; Gecen, N.; Sahin, K.; Yaamaz, E. Eur. J. Med. Chem. 2010, 45(9), 4157.
-
[14]
Sunduru, N.; Gupta, L.; Chaturvedi, V.; Dwivedi, R.; Sinh, S.; Chauhan, P. M. S. Eur. J. Med. Chem. 2010, 45(8), 3335. doi: 10.1016/j.ejmech.2010.04.017
-
[15]
Kong, D. X.; Yamori, I. Acta Pharmacol. Sin. 2010, 31, 1189. doi: 10.1038/aps.2010.150
-
[16]
Badr, S. M. I.; Barwa, R. M. Bioorg. Med. Chem. 2011, 19, 4506. doi: 10.1016/j.bmc.2011.06.024
-
[17]
Goswami, B. N.; Kattaky, J. C. S.; Baruah, J. N. J. Heterocycl. Chem. 1984, 21, 1225. doi: 10.1002/jhet.v21:4
-
[18]
Seelam, N.; Shrivastava, S. P.; Prasanthi, S.; Gupta, S. J. Saudi Chem. Soc. 2016, 20, 411. doi: 10.1016/j.jscs.2012.11.011
-
[19]
Papakonstantinou-Garoufalias, S. S.; Tani, E.; Todoulou, O.; Papadaki-Valiraki, A.; Filippatos, E.; De Clercq, E.; Kourounakis, P. N. J. Pharm. Pharmacol. 1998, 50, 117.
-
[20]
Turan-Zitouni, G.; Kaplancikli, Z. A.; Erol, K.; Kilic, F. S. Farmaco 1999, 54, 218. doi: 10.1016/S0014-827X(99)00016-6
-
[21]
Zhang, Z. Y.; Sun, X. W.; Chu, C. H.; Zhao, L. J. Chin. Chem. Soc. 1997, 44, 535. doi: 10.1002/jccs.v44.5
-
[22]
Amir, M.; Harish, K.; Javed, S. A. Eur. J. Med. Chem. 2008, 43, 2056. doi: 10.1016/j.ejmech.2007.09.025
-
[23]
Rzeski, W.; Matysiak, J.; Kandefer-Szerszen, M. Bioorg. Med. Chem. 2007, 15, 3201. doi: 10.1016/j.bmc.2007.02.041
-
[24]
Karabasanagouda, T.; Adhikari, A. V.; Shettey, N. S. Eur. J. Med. Chem. 2007, 42, 521. doi: 10.1016/j.ejmech.2006.10.010
-
[25]
Padmavathi, V.; Reddy, G. S.; Padmaja, A.; Kondaiah, P.; Shazia, A. Eur. J. Med. Chem. 2009, 44, 2106. doi: 10.1016/j.ejmech.2008.10.012
-
[26]
Shiradkar, M. R.; Padhalingappa, M. B.; Bhetalabhotala, S.; Akula, K. C.; Tupe, D. A.; Pinninti, R. R.; Thummanagoti, S. Bioorg. Med. Chem. 2007, 15, 6397. doi: 10.1016/j.bmc.2007.06.053
-
[27]
Webster, K. R.; Kimball, S. D.; Misra, R. N.; Xiao, H. Y.; Kim, K. S.; Lu, S. F.; Han, W. C.; Barbosa, S. A.; Hunt, J. T.; Rawlins, D. B.; Shan, W. F.; Ahmed, S. Z.; Qian, L. G.; Chen, B. C.; Zhao, R. L.; Bednarz, M. S.; Kellar, K. A.; Mulheron, J. G.; Batorsky, R.; Roongta, U.; Kamath, A.; Marathe, P.; Ranadive, S. A.; Sack, J. S.; Tokarski, J. S.; Pavletich, N. P.; Lee, F. Y. F. J. Med. Chem. 2004, 47, 1719. doi: 10.1021/jm0305568
-
[28]
Misra, R. N.; Xiao, H.; Y.; Williams, D. K.; Kim, K. S.; Lu, S. F.; Keller, K. A.; Mulheron, J. G.; Batorsky, R.; Tokarski, J. S.; Sack, J. S.; Kimball, S. D.; Lee, F. Y.; Webster, K. R. Bioorg. Med. Chem. Lett. 2004, 14, 2973. doi: 10.1016/j.bmcl.2004.02.105
-
[29]
Badr, S. M. I.; Barwa, R. M. Bioorg. Med. Chem., 2011, 19, 4506. doi: 10.1016/j.bmc.2011.06.024
-
[30]
Kumar, G. V. S.; Rajendraprasad, Y.; Mallikarjuna, B. P.; Chandrashekar, S. M.; Kistayya, C. Eur. J. Med. Chem. 2010, 45, 2063. doi: 10.1016/j.ejmech.2010.01.045
-
[31]
Sui, Z. H.; Guan, J.; Hlasta, D. J.; Macielag, M. J.; Foleno, B. D.; Goldschmidt, R. M.; Loeloff, M. J.; Webb, G. C.; Barrett, J. F. Bioorg. Med. Chem. Lett. 1998, 8, 1929. doi: 10.1016/S0960-894X(98)00325-4
-
[32]
Barton, B.; Gouwns, S.; Schaefer, M. C.; Zeelie, B. Org. Process Res. Dev. 2003, 7, 1071. doi: 10.1021/op0340715
-
[33]
Azenha, M. E. D.; Burrows, H. D.; Canle, M. L.; Coimbra, R.; Fernandez, M. I.; Garcıa, M. V.; Rodrigues, A. E.; Santaballa, J. A.; Steenken, S.; Santaballa, J. A. Chem. Commun. 2003, 112.
-
[34]
张成路, 国阳, 孙立杰, 武一菲, 朱长安, 曲瑞峰, 王雪, 柴金华, 应用化学, 2014, 31, 1419. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=yyhx201412010&dbname=CJFD&dbcode=CJFQZhang, C. L.; Guo, Y.; Sun, L. J.; Wu, Y. F.; Zhu, C. A.; Qu, R. F.; Wang, X.; Chai, J. H. Chin. J. Appl. Chem. 2014, 31, 1419(in Chinese). http://kns.cnki.net/KCMS/detail/detail.aspx?filename=yyhx201412010&dbname=CJFD&dbcode=CJFQ
-
[35]
张成路, 王雪, 胡雪, 孙立杰, 曲瑞峰, 国阳, 柴金华, 朱长安, 高等学校化学学报, 2015, 36, 463. http://www.cqvip.com/QK/90335X/201407/50252264.htmlZhang, C. L.; Wang, X; Hu, X.; Sun, L. J.; Qu, R. F.; Guo, Y.; Cai, J. H.; Zhu, C. A. Chem. J. Chin. Univ. 2015, 36, 463(in Chinese). http://www.cqvip.com/QK/90335X/201407/50252264.html
-
[36]
张成路, 唐杰, 殷立莹, 袭焕, 国阳, 孙立杰, 有机化学, 2016, 36, 358. http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract345260.shtmlZhang, C. L.; Tang, J.; Yin, L. Y.; Xi, H.; Guo, Y.; Sun, L. J. Chin. J. Org. Chem. 2016, 36, 358(in Chinese). http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract345260.shtml
-
[37]
Lavecchia, A.; Giovanni, C. D.; Pesapane, A.; Montuori, N.; Ragno, P.; Martucci, N. M.; Masullo, M.; Vendittis, E. D. J. Med. Chem. 2012, 55, 4142. doi: 10.1021/jm201624h
-
[38]
朱长安, 硕士论文, 辽宁师范大学, 大连, 2015.Zhu, C. A. M.S. Thesis, Liaoning Normal University, Dalian, 2015(in Chinese).
-
[39]
柴金华, 硕士论文, 辽宁师范大学, 大连, 2015.Chai, J. H. M.S. Thesis, Liaoning Normal University, Dalian, 2015(in Chinese).
-
[40]
孙立杰, 硕士论文, 辽宁师范大学, 大连, 2016.Sun, L. J. M.S. Thesis, Liaoning Normal University, Dalian, 2016(in Chinese).
-
[41]
张瑞波, 卢俊瑞, 辛春伟, 刘金彪, 穆江蓓, 杨旭云, 王宏韫, 王美君, 张贺, 有机化学, 2015, 35, 858. http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract344733.shtmlZhang, R. B.; Lu, J. R.; Xin, C. W.; Liu, J. B.; Mu, J. B.; Yang, X. Y.; Wang, H. Y.; Wang, M. J.; Zhang, H. Chin. J. Org. Chem. 2015, 35, 858(in Chinese). http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract344733.shtml
-
[42]
Wang, B. L.; Shi, Y. X.; Zhan, Y. Z.; Zhang, L. Y.; Zhang, Y.; Wang, L. Z.; Zhang, X.; Li, Y. H.; Li, Z. M.; Li, B. J. Chin. J. Chem. 2015, 33, 1124. doi: 10.1002/cjoc.201500436
-
[1]
-
图式 1 目标化合物1~8的合成方法
Scheme 1 Synthetic methods of target compounds 1~8
Reagents and condition: (a) (ⅰ) 0 ℃, CH2Cl2, (1) aniline, (2) p-chloroaniline, (3) morphine, (4) nafoxidine, (ⅱ) 10 ~ 20℃, NaOH-H2O, 2.5 h; (b) (ⅰ) 4 ~ 5 ℃, CH2Cl2, 0.5 h, (5) ally amine, (6) aniline, (7) p-chloroaniline, (8) morphine, (9) nafoxidine, (ⅱ) 10 ~ 20 ℃, NaOH-H2O, 2.5 h; (c) p-aminobenzoic acid, CH3COOH, 6 h; (d) THF, KOH, reflux, 8 h
表 1 目标分子(TM)对Cdc25B的抑制活性a
Table 1. Inhibitory activities of target molecules against Cdc25B
TM IC50/(μg/mL) 1 NAb 2 2.63±0.16 3 NA 4 NA 5 2.77±0.11 6 1.11±0.05 7 NA 8 NA 9 0.97±0.07 10 1.13±0.42 11 2.15±0.70 12 NA 13 2.23±0.74 14 NA 15 3.99±0.80 16 NA 17 2.46±0.12 18 2.40±0.11 19 0.44±0.07 20 0.72±0.27 21 0.56±0.06 1.25±0.14 Positive control: Na3VO4 was for Cdc25B a Value tested at 5 μg/mL concentration; bnot active at 5 μg/mL concentration (inhibition rate≤50%). -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 5
- 文章访问数: 832
- HTML全文浏览量: 164


 下载:
下载:



 下载:
下载:

