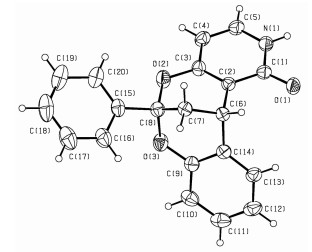
 图 1
化合物3r的晶体结构 (省去了H2O)
Figure 1.
X-ray structure of compound 3r(H2O is omitted for clarity)
图 1
化合物3r的晶体结构 (省去了H2O)
Figure 1.
X-ray structure of compound 3r(H2O is omitted for clarity)

Citation: Ye Mingyan, Qiu Shaozhong, Yin Guodong. Synthesis of Quinolinone-and Pyridinone-Fused Oxabicyclo[3.3.1]nonanes[J]. Chinese Journal of Organic Chemistry, 2017, 37(3): 667-674. doi: 10.6023/cjoc201609027

喹啉酮和吡啶酮稠合的氧杂双环[3.3.1]壬烷的合成
-
关键词:
- 4-羟基-2(1H)-喹啉酮
- / 4-羟基-2(1H)-吡啶酮
- / 双环[3.3.1]壬烷
- / 烷基化
- / 单晶结构
English
Synthesis of Quinolinone-and Pyridinone-Fused Oxabicyclo[3.3.1]nonanes
-
2(1H)-喹啉酮和2(1H)-吡啶酮是两类重要的杂环化合物, 其结构片段大量存在于许多天然的生物碱中, 如Euodenine A、Funiculosin、Ilicicolin H等[1~4].含有这类杂环骨架的分子常常展现出良好的抗菌、抗病毒和抗癌活性[5~7]. 4-羟基-2(1H)-喹啉酮是一个含有多官能团的分子, 羟基和氨基都容易参与反应, 3-位碳原子也具有很好的亲核性, 因此被广泛应用于合成一些功能化的杂环化合物[8~10].此外, 双环[3.3.1]壬烷是一种具有Ⅴ-型结构的迷人分子[11, 12], 它们在药物化学和分子识别中表现出潜在的应用价值[13, 14], 因此, 这类化合物的制备与功能化近年来受到了化学家的持续关注[15~20].在最近的研究中, 我们发展了一种以2-羟基查尔酮与六元环状的1, 3-二酮 (或萘酚/取代苯酚) 高立体选择性地合成2, 8-二氧杂双环[3.3.1]壬烷的新方法[21~23].然而, 目前鲜有文献引入含氮芳香环于此类结构中[24], 此处我们进一步将反应底物拓展为4-羟基-2(1H)-喹啉酮和4-羟基-2(1H)-吡啶酮衍生物, 立体选择性地合成了系列新型含有喹啉酮和吡啶酮稠合的2, 8-二氧杂双环[3.3.1]壬烷, 并发现前者与卤代烷烃反应, 喹啉酮片段可有效地转化为相应的氮烷基取代的喹啉酮和氧烷基取代的喹啉结构.
1 结果与讨论
在前面的工作中, 我们发现甲苯或正丙醇是合成香豆素稠合的2, 8-二氧杂双环[3.3.1]壬烷衍生物的理想溶剂[21].考虑到反应底物的溶解性, 我们将2-羟基查尔酮 (1a, 0.5 mmol) 和4-羟基-2(1H)-喹啉酮 (2a, 0.5 mmol) 混合于正丙醇中, 反应回流12 h后反应原料消失, 冷却至室温后, 发现有大量的白色固体从溶液中析出, 通过了1H NMR、13C NMR、IR和HRMS的表征, 证实其结构为8-苯基-2, 14-二氢-1H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉-1-酮 (3a).为了拓展该反应底物的范围, 我们尝试以各种取代的2-羟基查尔酮[25]与4-羟基-2(1H)-喹啉酮反应来考察该方法的通用性, 实验结果如Eq. 1所示.当底物1中R1为氢原子, R2为苯环上连接供电子取代基 (甲基、甲氧基), 相应的目标产物3b~3d可以较理想的收率分离得到.另外, 当R2为苯环上连接卤原子 (氟、氯和溴), 反应以68%~76%产率得到产物3e~3g.含有呋喃环、噻吩环和萘环取代的底物也适合该反应, 3h~3j的产率范围为65%~83%.当R2为甲基以及2-甲基-1-丙烯基时, 3k和3l分别以53%和51%的产率分离得到.若底物中R2为苯环或取代苯环, R1分别为4-溴、4-氯和2, 4-二氯取代的2-羟基查尔酮时, 产物3m~3o也可以65%~80%的产率得到.若R1和R3分别为氢原子和甲基, R2为苯环以及4-甲基取代苯环时, 则以73%和75%的产率分离得到3p和3q.
随后, 我们将底物4-羟基-2(1H)-喹啉酮拓展为一些代表性的2(1H)-吡啶酮, 观察到4-羟基-2(1H)-吡啶酮 (2b)、4-羟基-6-甲基-2(1H)-吡啶酮 (2c) 和4-羟基-5-乙酯基-2(1H)-吡啶酮 (2d) 也可以很好地与2-羟基查尔酮 (1a) 反应, 以较好的产率生成相应的产物3r~3t, 其中3r的结构得到了X射线单晶衍射的验证 (CCDC号为: 1505511), 如图 1所示. 4-羟基-5-氯-2(1H)-吡啶酮 (2e) 俗称吉莫斯特 (Gimeracil), 是一种抗肿瘤药物, 主要用于胃癌的治疗[26].我们发现, 在标准反应条件下, 2e与1a也可以生成目标产物3u.
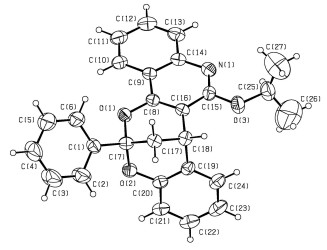
氧烷基取代的喹啉也是一种重要的分子, 含有这类结构片段的化合物大量存在于天然产物和具有良好生物活性分子中[27]. 2(1H)-喹啉酮中存在酰胺键, N原子上的氢很容易被取代, 我们也期望将上述得到的产物3a进一步衍生化.以碳酸钾为碱, 将溴代物4与3a混合于N, N-二甲基甲酰胺中反应, 反应过程中发生了O=CNH与C=NOH异构化[28].如Eq. 2所示, 当以溴乙烷和苄溴为烷基化试剂时, 喹啉酮片段均转化为两种结构, 分别为氮烷基取代的喹啉酮 (5a, 5b) 和氧烷基取代的喹啉 (6a, 6b), 且前者为主要产物[29].然而, 当溴代异丙烷和3a反应时, 只是分离得到了氧烷基取代的喹啉稠合双环[3.3.1]壬烷产物 (6c), 相应的氮烷基取代的产物5c并没有分离得到.可能是由于异丙基立体因素影响, 在形成氧烷基化时, 空间位阻更小的缘故. 6c的结构也获得了X射线单晶衍射的进一步证实 (CCDC号为1505510), 如图 2所示.
2 结论
本文报道了一种简单有效合成喹啉酮和吡啶酮稠合的2, 8-二氧杂双环[3.3.1]壬烷衍生物3的方法, 反应将易得的2-羟基查尔酮 (1) 与4-羟基-2(1H)-喹啉酮或4-羟基-2(1H)-吡啶酮 (2) 在正丙醇中回流既可以得到产物, 无需其它催化剂.药物分子4-羟基-5-氯-2(1H)-吡啶酮也成功地引入到目标产物中.我们将3a在碱性条件下与卤代烷烃反应, 将喹啉酮片段转化成了相应的烷氮基取代的喹啉酮和氧烷基取代的喹啉.本文中合成的目标产物均未见文献报道, 其结构都通过了1H NMR、13C NMR、IR和HRMS的表征, 其中3r和6c的结构和立体构型还获得了X射线单晶衍射的证实.
3 实验部分
3.1 仪器与试剂
熔点采用泰克X-4型数字显示显微熔点仪测定, 温度未经校正; 1H NMR和13C NMR采用Bruker AV 300型核磁共振仪测定, CDCl3或DMSO-d6为溶剂, TMS为内标; IR采用Nicolet 5700型红外分光光度仪测定 (KBr压片); HRMS采用Bruker UltrafleXtreme MALDI TOF/TOF[以α-氰基-4-羟基肉桂酸 (HCCA) 为基质]型高分辨质谱仪测定; X射线单晶衍射是通过Bruker SMART APEX CCD测定.
所用试剂均为市售分析纯, 未进一步纯化.所用有机溶剂在使用前均经过干燥并蒸馏提纯.
3.2 实验方法
3.2.2 目标化合物5/6的合成
将8-苯基-2, 14-二氢-1H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉-1-酮 (3a)(0.5 mmol) 和溴代物4 (1.5 mmol) 加入到25 mL耐压管中, 以3.0 equiv.的碳酸钾为碱, 再向其中加入N, N-二甲基甲酰胺 (DMF) (5 mL), 当反应物消失后 (3~8 h, 用TLC监测), 将混合物冷却至室温, 减压除去溶剂, 加入30 mL水后用乙酸乙酯萃取 (20 mL×3), 合并有机相后干燥、过滤, 再减压除去乙酸乙酯后, 剩余物以石油醚和乙酸乙酯为洗脱剂 (体积比为5:1~20:1), 通过硅胶柱层析分离得到目标化合物.
2-乙基-8-苯基-2, 14-二氢-1H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉-1-酮 (5a):白色固体119 mg, 产率60%. m.p. 210~212 ℃; 1H NMR (300 MHz, CDCl3) δ: 8.07 (dd, J=8.0, 1.2 Hz, 1H), 7.81~7.78 (m, 2H), 7.64~7.61 (m, 1H), 7.54~7.46 (m, 4H), 7.31 (d, J=8.5 Hz, 1H), 7.22~7.14 (m, 2H), 7.04 (d, J=7.7 Hz, 1H), 6.99~6.94 (m, 1H), 4.60~4.58 (m, 1H), 4.44~4.23 (m, 2H), 2.46~2.32 (m, 2H), 1.33 (t, J=7.1 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ: 161.1, 154.3, 151.8, 140.6, 137.5, 130.7, 129.1, 128.5, 128.3, 127.8, 126.1, 125.7, 123.2, 121.64, 121.61, 116.1, 115.6, 113.8, 110.9, 99.5, 37.3, 33.2, 27.3, 12.9; IR (KBr) ν: 3441, 1632, 1451, 1393, 1232, 1130, 892, 760, 696 cm-1; HRMS (MALDI) calcd for C26H22NO3 [M+H]+ 396.1594, found 396.1597.
2-苄基-8-苯基-2, 14-二氢-1H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉-1-酮 (5b):白色固体119 mg, 产率52%. m.p. 223~224 ℃; 1H NMR (300 MHz, CDCl3) δ: 8.07 (dd, J=1.3, 7.9 Hz, 1H), 7.81 (dd, J=1.8, 8.1 Hz, 2H), 7.63 (dd, J=1.5, 7.5 Hz, 1H), 7.54~7.46 (m, 3H), 7.41~7.36 (m, 2H), 7.31~7.26 (m, 2H), 7.22~7.14 (m, 5H), 7.06 (d, J=7.8 Hz, 1H), 7.00~6.97 (m, 1H), 5.74 (d, J=16.2 Hz, 1H), 5.32 (d, J=15.9 Hz, 1H), 4.66~4.64 (m, 1H), 2.50~2.37 (m, 2H); 13C NMR (75 MHz, CDCl3) δ: 161.9, 154.8, 151.8, 140.6, 138.1, 136.7, 130.8, 129.1, 128.7, 128.5, 128.3, 127.9, 127.1, 126.5, 126.1, 125.8, 123.1, 122.0, 121.8, 116.2, 115.7, 114.8, 110.9, 99.7, 46.1, 33.2, 27.4; IR (KBr) ν: 3431, 2923, 1632, 1597, 1454, 1131, 1006, 753, 697 cm-1; HRMS (MALDI) calcd for C31H24NO3 [M+H]+ 458.1751, found 458.1747.
1-乙氧基-8-苯基-14H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉 (6a):白色固体24 mg, 产率12%. m.p. 216~217 ℃; 1H NMR (300 MHz, CDCl3) δ: 8.13 (d, J=8.3 Hz, 1H), 7.84~7.81 (m, 2H), 7.73 (d, J=8.1 Hz, 1H), 7.58~7.44 (m, 5H), 7.33 (t, J=7.0 Hz, 1H), 7.18~7.12 (m, 1H), 7.04 (d, J=7.5 Hz, 1H), 6.96~6.92 (m, 1H), 4.66~4.56 (m, 2H), 4.55~4.53 (m, 1H), 2.49~2.38 (m, 2H), 1.57~1.53 (m, 3H); 13C NMR (75 MHz, CDCl3) δ: 159.6, 155.1, 152.1, 145.6, 140.9, 129.4, 129.0, 128.5, 127.9, 127.8, 126.8, 126.2, 125.8, 123.4, 121.6, 121.4, 118.2, 116.4, 106.8, 99.3, 62.0, 33.1, 27.0, 14.8; IR (KBr) ν: 3431, 2922, 1631, 1401, 1347, 1116, 887, 755, 696 cm-1; HRMS (MALDI) calcd for C26H22NO3 [M+H]+ 396.1594, found 396.1598.
1-苄氧基-8-苯基-14H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉 (6b):白色固体30 mg, 产率13%. m.p. 234~236 ℃; 1H NMR (300 MHz, CDCl3) δ: 8.15 (d, J=8.0 Hz, 1H), 7.83~7.76 (m, 3H), 7.60 (d, J=7.5 Hz, 3H), 7.48 (t, J=7.6 Hz, 4H), 7.33~7.30 (m, 4H), 7.13 (t, J=7.7 Hz, 1H), 7.03 (d, J=7.8 Hz, 1H), 6.86 (t, J=7.2 Hz, 1H), 5.56 (s, 2H), 4.54 (s, 1H), 2.41 (s, 2H); 13C NMR (75 MHz, CDCl3) δ: 159.3, 155.5, 152.1, 145.5, 140.9, 137.5, 129.6, 129.0, 128.5, 128.4, 127.9, 126.9, 126.0, 125.8, 123.6, 121.7, 121.4, 118.4, 116.4, 106.8, 99.4, 68.1, 33.1, 27.0; IR (KBr) ν: 3441, 1629, 1417, 1340, 1103, 999, 872, 760, 690 cm-1; HRMS (MALDI) calcd for C31H24NO3 [M+H]+ 458.1751, found 458.1749.
1-异丙氧基-8-苯基-14H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉 (6c):白色固体137 mg, 产率67%. m.p. 218~220 ℃; 1H NMR (300 MHz, CDCl3) δ: 8.05~8.02 (m, 1H), 7.75~7.72 (m, 2H), 7.63 (d, J=8.0 Hz, 1H), 7.48~7.42 (m, 3H), 7.40~7.37 (m, 1H), 7.25~7.20 (m, 1H), 7.15~7.03 (m, 2H), 6.96~6.94 (m, 1H), 6.88~6.82 (m, 1H), 5.59~5.51 (m, 1H), 4.44~4.42 (m, 1H), 2.34~2.32 (m, 2H) 1.44 (d, J=6.2 Hz, 3H), 1.37 (d, J=6.2 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ: 159.0, 155.1, 152.2, 145.6, 140.9, 129.3, 129.0, 128.5, 127.9, 127.8, 126.8, 126.2, 125.8, 123.2, 121.5, 121.3, 118.0, 116.4, 107.0, 99.2, 68.3, 33.0, 26.9, 22.4, 22.3; IR (KBr) ν: 3439, 2968, 1948, 1624, 1417, 1100, 876, 804, 753 cm-1; HRMS (MALDI) calcd for C27H24NO3 [M+H]+ 410.1751, found 410.1753.
辅助材料 (Supporting Information) 化合物3a~3u, 5a, 5b和6a~6c的1H NMR和13C NMR图谱, 3r和6c的晶体结构数据.这些材料可以免费从本刊网站 (http://sioc-journal.cn/) 上下载.
3.2.1 目标化合物3的合成
将2-羟基查尔酮类化合物1 (0.5 mmol) 和4-羟基-2(1H)-吡啶酮衍生物2 (0.5 mmol) 加入到25 mL耐压管中, 再向其中加入正丙醇 (5 mL), 反应一段时间后有大量固体析出.当反应物消失后[8~12 h, 用薄层色谱 (TLC) 监测], 将混合物冷却至室温, 固体用少量无水乙醇洗涤, 抽滤, 干燥, 得到目标化合物3a~3u.
8-苯基-2, 14-二氢-1H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉-1-酮 (3a):白色固体151 mg, 产率82%. m.p.>300 ℃; 1H NMR (300 MHz, DMSO-d6) δ: 11.63 (s, 1H), 7.83~7.80 (m, 3H), 7.57~7.42 (m, 5H), 7.30 (d, J=8.1 Hz, 1H), 7.22~7.14 (m, 2H), 7.02~6.92 (m, 2H), 4.39~4.37 (m, 1H), 2.47 (d, J=2.7 Hz, 2H); 13C NMR (75 MHz, DMSO-d6) δ: 161.0, 154.3, 151.4, 140.1, 137.3, 130.7, 129.1, 128.5, 127.8, 126.3, 125.6, 121.8, 121.7, 121.4, 115.9, 115.3, 113.5, 111.4, 99.4, 31.5, 26.0; IR (KBr) ν: 3417, 2950, 1653, 1492, 1398, 1233, 1035, 989, 753, 499 cm-1; HRMS (MALDI) calcd for C24H18NO3 [M+H]+368.1281, found 368.1280.
8-(4-甲基苯基)-2, 14-二氢-1H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉-1-酮 (3b):白色固体153 mg, 产率80%. m.p.>300 ℃; 1H NMR (300 MHz, DMSO-d6) δ: 11.62 (s, 1H), 7.80 (d, J=7.9 Hz, 1H), 7.69 (d, J=7.7 Hz, 2H), 7.53~7.42 (m, 2H), 7.35~7.29 (m, 3H), 7.18 (q, J=7.9 Hz, 2H), 7.01~6.92 (m, 2H), 4.37 (s, 1H), 2.50 (s, 2H), 2.38 (s, 3H); 13C NMR (75 MHz, DMSO-d6) δ: 161.0, 154.3, 151.4, 138.5, 137.32, 137.27, 130.6, 129.0, 127.8, 126.3, 125.5, 121.71, 121.68, 121.3, 115.8, 115.3, 113.5, 111.3, 99.4, 31.5, 26.1, 20.8; IR (KBr) ν: 3434, 2945, 1653, 1491, 1398, 1229, 1034, 992, 861, 750 cm-1; HRMS (MALDI) calcd for C25H20NO3 [M+H]+ 382.1438, found 382.1440.
8-(4-甲氧基苯基)-2, 14-二氢-1H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉-1-酮 (3c):白色固体157 mg, 产率79%. m.p.>300 ℃; 1H NMR (300 MHz, CDCl3) δ: 11.17 (s, 1H), 7.98 (d, J=7.4 Hz, 1H), 7.73 (d, J=8.9 Hz, 2H), 7.63 (dd, J=7.5, 1.3 Hz, 1H), 7.54~7.49 (m, 1H), 7.35 (d, J=8.1 Hz, 1H), 7.23~7.12 (m, 2H), 7.02 (d, J=8.8 Hz, 3H), 6.94 (t, J=7.4 Hz, 1H), 4.61~4.59 (m, 1H), 3.88 (s, 3H), 2.50~2.35 (m, 2H); 13C NMR (75 MHz, CDCl3) δ: 163.1, 160.2, 156.3, 151.8, 137.1, 132.8, 130.6, 128.1, 127.9, 127.1, 126.0, 122.6, 122.2, 121.6, 116.2, 115.7, 114.8, 113.8, 111.1, 99.7, 55.4, 33.1, 26.8; IR (KBr) ν: 3436, 2946, 1652, 1510, 1443, 1244, 1102, 1033, 999, 750 cm-1; HRMS (MALDI) calcd for C25H20NO4 [M+ H]+ 398.1387, found 398.1394.
8-(3, 4-二甲氧基苯基)-2, 14-二氢-1H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉-1-酮 (3d):白色固体160 mg, 产率75%. m.p.>300 ℃; 1H NMR (300 MHz, CDCl3) δ: 11.16 (s, 1H), 7.98 (d, J=8.1 Hz, 1H), 7.64 (d, J=6.3 Hz, 1H), 7.53 (t, J=7.1 Hz, 1H), 7.39~7.34 (m, 2H), 7.31 (d, J=2.0 Hz, 1H), 7.25~7.13 (m, 2H), 7.05~6.93 (m, 3H), 4.61 (s, 1H), 3.96 (s, 6H), 2.52~2.37 (m, 2H); 13C NMR (75 MHz, CDCl3) δ: 163.2, 156.3, 151.8, 149.7, 148.9, 137.1, 133.1, 130.6, 128.2, 127.9, 126.1, 122.5, 122.3, 121.7, 118.3, 116.2, 115.8, 114.8, 111.1, 110.8, 109.2, 99.7, 56.1, 56.0, 33.0, 26.8; IR (KBr) ν: 3430, 3001, 1653, 1513, 1345, 1133, 1098, 1025, 752 cm-1; HRMS (MALDI) calcd for C26H22NO5 [M+H]+ 428.1492, found 428.1492.
8-(4-氟苯基)-2, 14-二氢-1H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉-1-酮 (3e):白色固体131 mg, 产率68%. m.p.>300 ℃; 1H NMR (300 MHz, DMSO-d6) δ: 11.63 (s, 1H), 7.90~7.79 (m, 3H), 7.54~7.48 (m, 1H), 7.45 (d, J=1.4 Hz, 1H), 7.42~7.29 (m, 3H), 7.18 (q, J=7.4 Hz, 2H), 7.02~6.92 (m, 2H), 4.39~4.37 (m, 1H) 2.51 (s, 2H); 13C NMR (75 MHz, DMSO-d6) δ: 162.4 (d, 1JC-F=244.0 Hz), 160.9, 154.2, 151.3, 137.3, 136.5 (d, 4JC-F=3.0 Hz), 130.7, 128.1 (d, 3JC-F=8.9 Hz), 127.8, 126.2, 121.74, 121.69, 121.4, 115.8, 115.30 (d, 2JC-F=21.5 Hz), 115.26, 113.4, 111.4, 99.1, 31.3, 26.0; IR (KBr) ν: 3432, 2850, 1653, 1502, 1399, 1231, 1101, 1003, 835 cm-1; HRMS (MALDI) calcd for C24H17FNO3 [M+H]+ 386.1187, found 386.1186.
8-(4-氯苯基)-2, 14-二氢-1H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉-1-酮 (3f):白色固体153 mg, 产率76%. m.p.>300 ℃; 1H NMR (300 MHz, DMSO-d6) δ: 11.65 (s, 1H), 7.86~7.79 (m, 3H), 7.61 (d, J=8.4 Hz, 2H), 7.51 (t, J=7.6 Hz, 1H), 7.43 (d, J=7.4 Hz, 1H), 7.30 (d, J=8.2 Hz, 1H), 7.18 (q, J=8.1 Hz, 2H), 7.03~6.93 (m, 2H), 4.38 (s, 1H), 2.48 (s, 2H); 13C NMR (75 MHz, DMSO-d6) δ: 160.9, 154.2, 151.2, 139.1, 137.3, 133.9, 130.7, 128.6, 127.9, 127.8, 126.2, 121.8, 121.7, 121.5, 115.9, 115.3, 113.4, 111.4, 99.1, 31.2, 25.9; IR (KBr) ν: 3727, 3424, 2860, 1663, 1435, 1328, 1094, 881, 863 cm-1; HRMS (MALDI) calcd for C24H17ClNO3 [M+H]+ 402.0891, found 402.0890.
8-(4-溴苯基)-2, 14-二氢-1H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉-1-酮 (3g):白色固体161 mg, 产率72%. m.p.>300 ℃; 1H NMR (300 MHz, DMSO-d6) δ: 11.63 (s, 1H), 7.82~7.73 (m, 5H), 7.53~7.43 (m, 2H), 7.32~7.29 (m, 1H), 7.22~7.17 (m, 2H), 7.02~6.95 (m, 2H), 4.38 (s, 1H), 2.50~2.48 (m, 2H); 13C NMR (75 MHz, DMSO-d6) δ: 160.9, 154.1, 151.2, 139.5, 137.3, 131.4, 130.6, 128.0, 127.8, 126.2, 122.5, 121.71, 121.66, 121.4, 115.8, 115.2, 113.4, 111.3, 99.1, 31.1, 25.9; IR (KBr) ν: 3442, 2850, 1651, 1489, 1394, 1230, 1101, 1034, 749 cm-1; HRMS (MALDI) calcd for C24H17BrNO [M+H]+ 446.0386, found 446.0392.
8-(2-呋喃基)-2, 14-二氢-1H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉-1-酮 (3h):白色固体115 mg, 产率65%. m.p.>300 ℃; 1H NMR (300 MHz, DMSO-d6) δ: 11.65 (s, 1H), 7.85~7.79 (m, 2H), 7.53~7.44 (m, 1H), 7.41 (d, J=1.2 Hz, 1H), 7.29 (d, J=8.1Hz, 1H), 7.22~7.12 (m, 2H), 7.00~6.92 (m, 3H), 6.64~6.63 (m, 1H), 4.41~4.40 (m, 1H), 2.62~2.57 (m, 2H); 13C NMR (75 MHz, DMSO-d6) δ: 160.8, 153.6, 151.0, 150.8, 143.8, 137.2, 130.7, 127.9, 126.1, 121.8, 121.5, 115.8, 115.3, 113.2, 111.4, 110.7, 108.2, 95.7, 28.7, 25.2; IR (KBr) ν: 3428, 2850, 2357, 1654, 1397, 1103, 961, 884, 750 cm-1; HRMS (MALDI) calcd for C22H16NO4 [M+H]+ 358.1074, found 358.1072.
8-(2-噻吩基)-2, 14-二氢-1H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉-1-酮 (3i):白色固体125 mg, 产率67%. m.p.>300 ℃; 1H NMR (300 MHz, DMSO-d6) δ: 11.65 (d, J=1.9 Hz, 1H), 7.77 (d, J=8.0 Hz, 1H), 7.71 (d, J=3.8 Hz, 1H), 7.70~7.48 (m, 2H), 7.43 (d, J=7.8 Hz, 1H), 7.31~7.28 (m, 1H), 7.23~7.13 (m, 3H), 6.97~6.93 (m, 2H), 4.40 (s, 1H), 2.71~2.61 (m, 2H); 13C NMR (75 MHz, DMSO-d6) δ: 160.9, 153.9, 151.0, 143.1, 137.3, 130.8, 127.9, 127.3, 127.0, 126.1, 125.8, 121.9, 121.6, 115.9, 115.4, 113.3, 111.5, 98.4, 31.5, 26.0; IR (KBr) ν: 3434, 2852, 1651, 1492, 1397, 1101, 986, 877, 755 cm-1; HRMS (MALDI) calcd for C22H16NO3S [M+H]+ 374.0845, found 374.0842.
8-(1-萘基)-2, 14-二氢-1H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉-1-酮 (3j):白色固体173 mg, 产率83%. m.p.>300 ℃; 1H NMR (300 MHz, CDCl3) δ: 10.86 (s, 1H), 8.41 (d, J=8.3 Hz, 1H), 8.11 (d, J=6.5 Hz, 1H), 7.99~7.95 (m, 2H), 7.91 (d, J=7.4 Hz, 1H), 7.70 (d, J=1.4 Hz, 1H), 7.68~7.58 (m, 2H), 7.55~7.47 (m, 2H), 7.34 (d, J=8.1 Hz, 1H), 7.21~7.15 (m, 2H), 7.04~6.97 (m, 2H), 4.71~4.69 (m, 1H), 2.87~2.72 (m, 2H); 13C NMR (75 MHz, CDCl3) δ: 163.3, 155.9, 151.4, 137.2, 135.3, 134.8, 130.7, 130.5, 129.1, 128.2, 128.1, 126.4, 125.74, 125.66, 124.8, 124.3, 122.7, 122.3, 121.8, 116.4, 115.8, 114.8, 111.4, 100.8, 31.0, 26.4; IR (KBr) ν: 3407, 2853, 1650, 1495, 1398, 1290, 1113, 938, 750 cm-1; HRMS (MALDI) calcd for C28H20NO3 [M+H]+ 418.1438, found 418.1437.
8-甲基-2, 14-二氢-1H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉-1-酮 (3k):白色固体81 mg, 产率53%. m.p.>300 ℃; 1H NMR (300 MHz, CDCl3) δ: 10.16 (s, 1H), 7.91 (d, J=8.2 Hz, 1H), 7.54 (d, J=7.6 Hz, 1H), 7.50~7.44 (m, 1H), 7.22~7.16 (m, 2H), 7.11~7.05 (m, 1H), 6.91~6.85 (m, 2H), 4.50~4.48 (m, 1H), 2.34~2.18 (m, 2H), 2.00 (s, 3H); 13C NMR (75 MHz, CDCl3) δ: 162.6, 156.1, 151.5, 136.7, 130.5, 128.1, 127.7, 125.9, 122.7, 122.1, 121.3, 115.9, 115.2, 114.7, 111.0, 99.0, 30.8, 26.9, 26.2; IR (KBr) ν: 3432, 3065, 2942, 1653, 1489, 1447, 1149, 1086, 809 cm-1; HRMS (MALDI) calcd for C19H16NO3: [M+H]+ 306.1125, found 306.1123.
8-(2-甲基-1-丙烯基)-2, 14-二氢-1H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉-1-酮 (3l):白色固体88 mg, 产率51%. m.p.>300 ℃; 1H NMR (300 MHz, CDCl3) δ: 10.68 (s, 1H), 7.93 (d, J=0.9 Hz, 1H), 7.91 (d, J=1.0 Hz, 1H), 7.57~7.50 (m, 1H), 7.28 (s, 1H), 7.19 (t, J=8.0 Hz, 1H), 7.12~7.07 (m, 1H), 6.90 (t, J=7.7 Hz, 2H), 5.80 (s, 1H), 4.50~4.48 (m, 1H), 2.39~2.23 (m, 2H), 1.92 (s, 6H); 13C NMR (75 MHz, CDCl3) δ: 163.3, 156.1, 151.5, 140.7, 137.1, 130.4, 128.1, 127.8, 126.2, 124.2, 122.6, 122.1, 121.4, 116.1, 115.7, 114.8, 111.0, 99.1, 30.5, 26.9, 26.2, 19.3; IR (KBr) ν: 3436, 2852, 1649, 1441, 1399, 1100, 957, 880, 752 cm-1; HRMS (MALDI) calcd for C22H20NO3 [M+H]+ 346.1438, found 346.1432.
12-溴-8-苯基-2, 14-二氢-1H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉-1-酮 (3m):白色固体176 mg, 产率79%. m.p.>300 ℃; 1H NMR (300 MHz, DMSO-d6) δ: 11.69 (s, 1H), 7.83~7.81 (m, 3H), 7.58~7.49 (m, 5H), 7.36~7.31 (m, 2H), 7.22 (t, J=7.6 Hz, 1H), 7.01 (d, J=8.7 Hz, 1H), 4.38 (s, 1H), 2.51 (s, 2H); 13C NMR (75 MHz, DMSO-d6) δ: 160.9, 154.4, 150.9, 139.7, 137.4, 130.9, 130.4, 129.9, 129.3, 128.9, 128.6, 125.7, 121.9, 121.8, 118.2, 115.4, 113.4, 112.6, 110.7, 99.6, 30.9, 25.9; IR (KBr) ν: 3434, 2818, 1652, 1488, 1402, 1243, 1102, 949, 753 cm-1; HRMS (MALDI) calcd for C24H17-BrNO3 [M+H]+ 446.0386, found 446.0391.
12-氯-8-(4-甲氧基苯基)-2, 14-二氢-1H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉-1-酮 (3n):白色固体172 mg, 产率80%. m.p.>300 ℃; 1H NMR (300 MHz, DMSO-d6) δ: 11.65 (s, 1H), 7.81~7.72 (m, 3H), 7.52~7.49 (m, 1H), 7.41 (s, 1H), 7.32 (d, J=8.2 Hz, 1H), 7.20 (s, 2H), 7.08~7.02 (m, 3H), 4.36 (s, 1H), 3.82 (s, 3H), 2.47 (s, 2H); 13C NMR (75 MHz, DMSO-d6) δ: 160.9, 159.8, 154.5, 150.5, 137.3, 131.9, 130.8, 128.4, 127.5, 127.0, 124.7, 121.8, 121.7, 117.7, 115.3, 113.8, 113.4, 110.6, 99.6, 55.2, 31.0, 26.1; IR (KBr) ν: 2948, 2841, 1652, 1510, 1242, 1028, 1002, 821, 755 cm-1; HRMS (MALDI) calcd for C25H19ClNO4 [M+H]+ 432.0997, found 432.0999.
10, 12-二氯-8-(4-氟苯基)-2, 14-二氢-1H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉-1-酮 (3o):白色固体148 mg, 产率65%. m.p.>300 ℃; 1H NMR (300 MHz, DMSO-d6) δ: 11.74 (s, 1H), 7.90 (t, J=7.3 Hz, 2H), 7.81 (d, J=7.9 Hz, 1H), 7.57~7.51 (m, 2H), 7.43~7.32 (m, 4H), 7.24~7.19 (m, 1H), 4.43 (s, 1H), 2.65~2.46 (m, 2H); 13C NMR (75 MHz, DMSO-d6) δ: 162.6 (d, 1JC-F=244.5 Hz), 160.8, 154.2, 146.4, 137.4, 135.5 (d, 4JC-F=2.9 Hz), 131.0, 129.7, 128.2 (d, 3JC-F=8.6 Hz), 127.5, 126.1, 125.0, 121.9, 121.8, 120.9, 115.46 (d, 2JC-F=21.6 Hz), 115.38, 113.2, 110.0, 99.9, 30.5, 26.2; IR (KBr) ν: 3498, 2947, 2411, 1652, 1507, 1292, 1099, 834, 752 cm-1; HRMS (MALDI) calcd for C24H15Cl2FNO3 [M+H]+ 454.0408, found 454.0398.
2-甲基-8-苯基-2, 14-二氢-1H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉-1-酮 (3p):白色固体143 mg, 产率73%. m.p. 185~186 ℃; 1H NMR (300 MHz, CDCl3) δ: 8.09~8.06 (m, 1H), 7.82~7.78 (m, 2H), 7.63~7.60 (m, 1H), 7.58~7.46 (m, 4H), 7.32 (d, J=8.5 Hz, 1H), 7.23~7.13 (m, 2H), 7.05~6.93 (m, 2H), 4.60~4.58 (m, 1H), 3.71 (s, 3H), 2.48~2.33 (m, 2H); 13C NMR (75 MHz, CDCl3) δ: 161.7, 154.4, 151.8, 140.6, 138.6, 130.8, 129.1, 128.5, 128.2, 127.9, 126.1, 125.8, 123.1, 121.9, 121.7, 116.1, 115.5, 114.0, 111.1, 99.5, 33.2, 29.4, 27.4; IR (KBr) ν: 3434, 1638, 1490, 1397, 1271, 1116, 1038, 959, 751 cm-1; HRMS (MALDI) calcd for C25H20NO3 [M+H]+ 382.1438, found 382.1438.
2-甲基-8-(4-甲基苯基)-2, 14-二氢-1H-8, 14-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]喹啉-1-酮 (3q):白色固体148 mg, 产率75%. m.p. 191~192 ℃; 1H NMR (300 MHz, CDCl3) δ: 8.06 (d, J=8.1 Hz, 1H), 7.68 (d, J=7.8 Hz, 2H), 7.61 (d, J=7.0 Hz, 1H), 7.54 (t, J=8.6 Hz, 1H), 7.31 (d, J=7.8 Hz, 3H), 7.22~7.13 (m, 2H), 7.04~6.93 (m, 2H), 4.58 (s, 1H), 3.70 (s, 3H), 2.43~2.32 (m, 5H); 13C NMR (75 MHz, CDCl3) δ: 161.7, 154.5, 151.9, 139.0, 138.6, 137.7, 130.8, 129.2, 128.2, 127.9, 126.1, 125.7, 123.1, 121.8, 121.6, 116.1, 115.5, 113.9, 111.0, 99.6, 33.2, 29.4, 27.4, 21.2; IR (KBr) ν: 3434, 1636, 1466, 1395, 1270, 1180, 1004, 956, 755 cm-1; HRMS (MALDI) calcd for C26H22NO3 [M+H]+ 396.1594, found 396.1595.
6-苯基-2, 12-二氢-1H-6, 12-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]吡啶-1-酮 (3r):白色固体124 mg, 产率78%. m.p. 246~248 ℃; 1H NMR (300 MHz, CDCl3) δ: 11.28 (s, 1H), 7.73~7.69 (m, 2H), 7.55~7.52 (m, 1H), 7.50~7.42 (m, 3H), 7.18~7.12 (m, 2H), 7.03 (d, J=8.1 Hz, 1H), 6.96~6.91 (m, 1H), 6.12~6.09 (m, 1H), 4.43~4.42 (m, 1H), 2.40~2.26 (m, 2H); 13C NMR (75 MHz, CDCl3) δ: 163.7, 161.2, 151.7, 140.3, 132.5, 129.1, 128.4, 128.0, 127.9, 126.1, 125.6, 121.6, 116.2, 112.2, 100.8, 99.4, 32.7, 26.4; IR (KBr) ν: 3436, 2361, 1642, 1558, 1465, 1119, 1004, 1029, 755 cm-1; HRMS (MALDI) calcd for C20H16-NO3 [M+H]+ 318.1125, found 318.1123.
3-甲基-6-苯基-2, 12-二氢-1H-6, 12-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]吡啶-1-酮 (3s):白色固体113 mg, 产率68%. m.p. 277~279 ℃; 1H NMR (300 MHz, CDCl3) δ: 12.19 (s, 1H), 7.72~7.69 (m, 2H), 7.51~7.42 (m, 4H), 7.16~7.10 (m, 1H), 7.01 (d, J=7.5 Hz, 1H), 6.92~6.86 (m, 1H), 5.90 (s, 1H), 4.39~4.37 (m, 1H), 2.32~2.28 (m, 5H); 13C NMR (75 MHz, CDCl3) δ: 164.2, 161.4, 151.8, 143.6, 140.5, 129.0, 128.4, 127.8, 127.7, 126.5, 125.6, 121.4, 116.2, 108.7, 99.4, 99.3, 33.0, 26.3, 19.0; IR (KBr) ν: 3423, 2361, 1632, 1449, 1255, 1178, 1109, 1026, 797, 757 cm-1; HRMS (MALDI) calcd for C21H18NO3 [M+H]+ 332.1281, found 332.1280.
4-乙酯基-6-苯基-2, 12-二氢-1H-6, 12-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]吡啶-1-酮 (3t):白色固体148 mg, 产率76%. m.p. 244~246 ℃; 1H NMR (300 MHz, CDCl3) δ: 12.09 (s, 1H), 8.11 (s, 1H), 7.87~7.84 (m, 2H), 7.55~7.44 (m, 4H), 7.21~7.15 (m, 1H), 7.03~6.93 (m, 2H), 4.46~4.44 (m, 1H), 4.41~4.26 (m, 2H), 2.51~2.22 (m, 2H), 1.35 (t, J=7.1 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ: 163.5, 163.3, 159.5, 151.7, 140.1, 139.3, 129.1, 128.4, 128.1, 128.0, 126.0, 125.5, 121.7, 116.3, 112.1, 105.3, 99.9, 61.1, 32.2, 26.4, 14.3; IR (KBr) ν: 2857, 2363, 1726, 1637, 1440, 1285 cm-1; HRMS (MALDI) calcd for C23H20NO5 [M+H]+ 390.1336, found 390.1341.
4-氯-6-苯基-2, 12-二氢-1H-6, 12-亚甲基苯并[7,8][1,3]二氧桥[5, 4-c]吡啶-1-酮 (3u):白色固体114 mg, 产率65%. m.p.>300 ℃; 1H NMR (300 MHz, DMSO-d6) δ: 11.69 (s, 1H), 7.75~7.72 (m, 2H), 7.55~7.47 (m, 4H), 7.39 (dd, J=7.5, 1.5 Hz, 1H), 7.21~7.15 (m, 1H), 7.02~6.92 (m, 2H), 4.28~4.26 (m, 1H), 2.47~2.34 (m, 2H); 13C NMR (75 MHz, DMSO-d6) δ: 160.3, 155.1, 151.1, 139.7, 132.1, 129.1, 128.4, 127.9, 127.7, 126.0, 125.5, 121.4, 115.9, 112.5, 103.3, 99.6, 31.1, 26.1; IR (KBr) ν: 3434, 2760, 1651, 1483, 1246, 1163, 1076, 928, 757 cm-1; HRMS (MALDI) calcd for C20H15ClNO3 [M+H]+ 352.0735, found 352.0731.
-
-
[1]
Neve, J. E.; Wijesekera, H. P.; Duffy, S.; Jenkins, I. D.; Ripper, J. A.; Teague, S. J.; Campitelli, M.; Garavelas, A.; Nikolakopoulos, G.; Le, P. V.; de A. Leone, P.; Pham, N. B.; Shelton, P.; Fraser, N.; Carroll, A. R.; Avery, V. M.; McCrae, C.; Williams, N.; Quinn, R. J. J. Med. Chem. 2014, 57, 1252. doi: 10.1021/jm401321v
-
[2]
赵圣印, 黄婧, 程健, 刘保硕, 陈晨, 有机化学, 2012, 32, 651. doi: 10.6023/cjoc1109192Zhao, S.; Huang, J.; Cheng, J.; Liu, B.; Chen, C. Chin. J. Org. Chem. 2012, 32, 651 (in Chinese). doi: 10.6023/cjoc1109192
-
[3]
Banbury, L. K.; Shou, Q.; Renshaw, D. E.; Lambley, E. H.; Griesser, H. J.; Mon H.; Wohlmuth, H. J. Ethnopharmacol. 2015, 163, 251. doi: 10.1016/j.jep.2015.01.033
-
[4]
Ahsan, M.; Haque, M. R.; Hossain, M. B.; Islam, S. N.; Gray, A. I.; Hasan, C. M. Phytochemistry 2014, 103, 8. doi: 10.1016/j.phytochem.2014.03.008
-
[5]
Tedesco, R.; Shaw, A. N.; Bambal, R.; Chai, D.; Concha, N. O.; Darcy, M. G.; Dhanak, D.; Fitch, D. M.; Gates, A.; Gerhardt, W. G.; Halegoua, D. L.; Han, C.; Hofmann, G. A.; Johnston, V. K.; Kaura, A. C.; Liu, N.; Keenan, R. M.; Lin-Goerke, J.; Sarisky, R. T.; Wiggall, K. J.; Zimmerman, M. N.; Duffy, K. J. J. Med. Chem. 2006, 49, 971. doi: 10.1021/jm050855s
-
[6]
Chen, T.; Luo, Y.; Hu, Y.; Yang, B.; Lu, W. Eur. J. Med. Chem. 2013, 64, 613. doi: 10.1016/j.ejmech.2013.04.008
-
[7]
Detsi, Α.; Bouloumbasi, D.; Prousis, K. C.; Κoufaki, Μ.; Athanasellis, G.; Melagraki, G.; Afantitis, A.; Lgglessi-Markopoulou, O.; Kontogiorgis, C.; Hadjipavlou-Litina, D. J. J. Med. Chem. 2007, 50, 2450. doi: 10.1021/jm061173n
-
[8]
Nishino, H.; Kumabe, R.; Hamada, R.; Yakut, M. Tetrahedron 2014, 70, 1437. doi: 10.1016/j.tet.2014.01.013
-
[9]
Lei, M.; Ma, L.; Hu, L. Tetrahedron Lett. 2011, 52, 2597. doi: 10.1016/j.tetlet.2011.03.061
-
[10]
裴文, 邓琼, 王海滨, 孙莉, 有机化学, 2006, 26, 364. http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract333050.shtmlPei, W.; Deng, Q.; Wang, H.; Sun L. Chin. J. Org. Chem. 2006, 26, 364 (in Chinese). http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract333050.shtml
-
[11]
Presset, M.; Coquerel, Y.; Rodriguez, J. Chem. Rev. 2013, 113, 525. doi: 10.1021/cr200364p
-
[12]
Ruiz, M.; López-Alvarado, P.; Giorgi, G.; Menéndez, J. C. Chem. Soc. Rev. 2011, 40, 3445. doi: 10.1039/c1cs15018a
-
[13]
Chen, D.; Kuo, P.; Yang, D. Bioorg. Med. Chem. Lett. 2005, 15, 2665. doi: 10.1016/j.bmcl.2005.03.005
-
[14]
He, Z.; Yang, X.; Jiang, W. Org. Lett. 2015, 17, 3880. doi: 10.1021/acs.orglett.5b01871
-
[15]
Wang, F.; Chen, F.; Qu, M.; Li, T.; Liu, Y.; Shi, M. Chem. Commun. 2013, 49, 3360. doi: 10.1039/c3cc00295k
-
[16]
Reddy, G. M.; Sridhar, P. R. Eur. J. Org. Chem. 2014, 1496.
-
[17]
Xia, L.; Cai, H.; Lee, Y. R. Org. Biomol. Chem. 2014, 12, 4386. doi: 10.1039/c4ob00691g
-
[18]
Jiang, X.; Song, Z.; Xu, C.; Yao, Q.; Zhang, A. Eur. J. Org. Chem. 2014, 418.
-
[19]
Srinivas, V.; Koketsu, M. J. Org. Chem. 2013, 78, 11612. doi: 10.1021/jo401990h
-
[20]
Ganguly, N. C.; Roy, S.; Mondal, P. RSC Adv. 2014, 4, 42078. doi: 10.1039/C4RA05927A
-
[21]
Yin, G.; Ren, T.; Rao, Y.; Zhou, Y.; Li, Z.; Shu, W.; Wu, A. J. Org. Chem. 2013, 78, 3132. doi: 10.1021/jo400081q
-
[22]
Rao, Y.; Yin, G. Org. Biomol. Chem. 2013, 11, 6029. doi: 10.1039/c3ob40860d
-
[23]
Rao, Y.; Liu, M.; Wu, L.; Yin, G. RSC Adv. 2014, 4, 64551. doi: 10.1039/C4RA13166E
-
[24]
Bingi, C.; Emmadi, N. R.; Chennapuram, M.; Nanubolu, J. B.; Atmakur, K. RSC Adv. 2014, 4, 35009. doi: 10.1039/C4RA07278B
-
[25]
Yin, G.; Fan, L.; Ren, T.; Zheng, C.; Tao, Q.; Wu, A.; She, N. Org. Biomol. Chem. 2012, 10, 8877. doi: 10.1039/c2ob26642c
-
[26]
Kinoshita, T.; Nashimoto, A.; Yamamura, Y.; Okamura, T.; Sasako, M.; Sakamoto, J.; Kojima, H.; Hiratsuka, M.; Arai, K.; Sairenji, M.; Fukushima, N.; Kimura, H.; Nakajima, T. Gastric Cancer 2004, 7, 104.
-
[27]
Luiza, B. D. O.; Borgati, T. F.; de Freitas, R. P.; Ruiz, A. L.; Marchetti, G. M.; de Carvalho, J. E.; da Cunha, E. F. F.; Ramalho, T. C.; Alves, R. B. Eur. J. Med. Chem. 2014, 84, 595. doi: 10.1016/j.ejmech.2014.07.061
-
[28]
Hao, X.; Xu, Z.; Lu, H.; Dai, X.; Yang, T.; Lin, X.; Ren, F. Org. Lett. 2015, 17, 3382. doi: 10.1021/acs.orglett.5b01628
-
[29]
Torhan, M. C.; Peet, N. P.; Williams, J. D. Tetrahedron Lett. 2013, 54, 3926. doi: 10.1016/j.tetlet.2013.05.054
-
[1]
-

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 5
- 文章访问数: 1484
- HTML全文浏览量: 186


 下载:
下载:

 下载:
下载:

