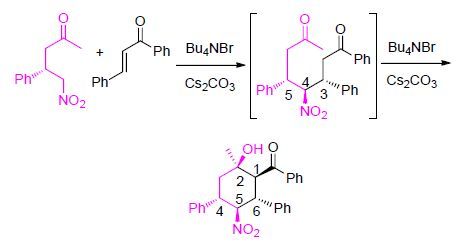
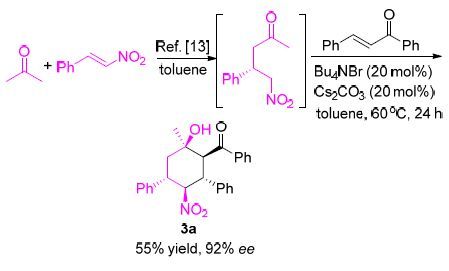
-
[1]
For selected reviews, see: (a) Tietze, L. F. Chem. Rev. 1996, 96, 115. (b) Parsons, P. J.; Penkett, C. S.; Shell, A. J. Chem. Rev. 1996, 96, 195. (c) Pellissier, H. Tetrahedron 2006, 62, 1619. (d) Pellissier, H. Tetrahedron 2006, 62, 2143. (e) Nicolaou, K. C.; Edmonds, D. J.; Bulger, P. G. Angew. Chem., Int. Ed. 2006, 45, 7134. (f) Chapman, C. J.; Frost, C. G. Synthesis 2007, 1. (g) Enders, D.; Grondal, C.; Hüttl, M. R. M. Angew. Chem., Int. Ed. 2007, 46, 1570. (h) Nicolaou, K. C.; Chen, J. S. Chem. Soc. Rev. 2009, 38, 2993. (i) Grondal, C.; Jeanty, M.; Enders, D. Nat. Chem. 2010, 2, 167. (j) Ruiz, M.; López-Alvarado, P.; Giorgi, G., Menéndez, J. C. Chem. Soc. Rev. 2011, 40, 3445. (k) Albrecht, Ł.; Jiang, H.; Jørgensen, K. A. Angew. Chem., Int. Ed. 2011, 50, 8492. (l) Pellissier, H. Adv. Synth. Catal. 2012, 354, 237. (m) Pellissier, H. Chem. Rev. 2013, 113, 442. (n) Volla, C. M. R.; Atodiresei, I.; Rueping, M. Chem. Rev. 2014, 114, 2390. (o) Vetica, F.; de Figueiredo, R. M.; Orsini, M.; Tofani, D.; Gasperi, T. Synthesis 2015, 47, 2139.
-
[2]
Merino, P.; Marqués-López, E.; Tejero, T.; Herrera, R. P. Synthesis 2010, 1. (b) Moyano, A.; Rios, R. Chem. Rev. 2011, 111, 4703. (c) Pellissier, H. Tetrahedron 2012, 68, 2197. (d) Xie, P.; Huang, Y. Eur. J. Org. Chem. 2013, 6213. (e) Yang, X.; Wang, J.; Li, P. Org. Biomol. Chem. 2014, 12, 2499.
-
[3]
Posner, G. H. Chem. Rev. 1986, 86, 831. (b) Schultz, A. G. Acc. Chem. Res. 1990, 23, 207. (c) Li J. K. In Name Reaction for Carbocyclic Ring Formarions, Wiley, Hoboken, NJ, 2010, p. 197-142. (d) Goudedranche, S.; Raimondi, W.; Bugaut, X.; Constantieux, T.; Bonne, D.; Rodriguez, J. Synthesis 2013, 45, 1909.
-
[4]
Enders, D.; Hüttl, M. R. M.; Grondal, C.; Raabe, G. Nature 2006, 441, 861. (b) Enders, D.; Hüttl, M. R. M.; Runsink, J.; Raabe, G.; Wendt, B. Angew. Chem., Int. Ed. 2007, 46, 467. (c) Enders, D.; Hüttl, M. R. M.; Raabe, G.; Bats, J. W. Adv. Synth. Catal. 2008, 350, 267. (d) Ishikawa, H.; Suzuki, T.; Orita, H.; Uchimaru, T.; Hayashi, Y. Chem. Eur. J. 2010, 16, 12616. (e) Anwar, S.; Chang, H.-J.; Chen, K. Org. Lett. 2011, 13, 2200. (f) Ma, G.; Lin, S.; Ibrahem, I.; Kubik, G.; Liu, L.; Sun, J.; Córdova, A. Adv. Synth. Catal. 2012, 354, 2865.
-
[5]
Han, B.; Xiao, Y.-C.; He, Z.-Q.; Chen, Y.-C. Org. Lett. 2009, 11, 4660. (b) Wang, Y.; Yu, D.-F.; Liu, Y.-Z.; Wei, H.; Luo, Y.-C.; Dixon, D. J.; Xu, P.-F. Chem. Eur. J. 2010, 16, 3922.
-
[6]
Ishikawa, H.; Sawano, S.; Yasui, Y.; Shibata, Y.; Hayashi, Y. Angew. Chem., Int. Ed. 2011, 50, 3774. (b) Hahn, R.; Raabe, G.; Enders, D. Org. Lett. 2014, 16, 3636.
-
[7]
Mao, Z.; Jia, Y.; Xu, Z.; Wang, R. Adv. Synth. Catal. 2012, 354, 1401. (b) Enders, D.; Hahn, R.; Atodiresei, I. Adv. Synth. Catal. 2013, 355, 1126. (c) Chauhan, P.; Mahajan, S.; Loh, C. C. J.; Raabe, G.; Enders, D. Org. Lett. 2014, 16, 2954. (d) Chauhan, P.; Urbanietz, G.; Raabe, G.; Enders, D. Chem. Commun. 2014, 50, 6853. (e) Zhou, R.; Wu, Q.; Guo, M.; Huang, W.; He, X.; Yang, L.; Peng, F.; He, G.; Han, B. Chem. Commun. 2015, 51, 13113.
-
[8]
Xie, X.; Peng, C.; He, G.; Leng, H.-J.; Wang, B.; Huang, W.; Han, B. Chem. Commun. 2012, 48, 10487.
-
[9]
Varga, S.; Jakab, G.; Drahos, L.; Holczbauer, T.; Czugler, M.; Soós, T. Org. Lett. 2011, 13, 5416. (b) Sun, J.; Jiang, C.; Zhou, Z. Eur. J. Org. Chem. 2016, 1165.
-
[10]
Duan, J.-D.; Cheng, J.; Li, P.-F. Org. Chem. Front. 2015, 2, 1048.
-
[11]
Yu, L.; Yang, Z.; Peng, J.-Z.; Li, P.-F. Eur. J. Org. Chem. 2016, 535.
-
[12]
Yu, L.; Yang, Q.-J.; Li, P.-F. Eur. J. Org. Chem. 2014, 7499.
-
[13]
Huang, H.; Jacobsen, E. N. J. Am. Chem. Soc. 2006, 128, 7170.

 Login In
Login In







 DownLoad:
DownLoad: