
Citation: Zhou Peng, Qiu Huihua, Pan Hongcheng, Shi Jicheng, Zhou Jianmin. Researches on the α-Aminoxylation between α-Azoleketones and 2,2,6,6-Tetramethylpiperidine-1-oxyl with Cu/O2[J]. Chinese Journal of Organic Chemistry, 2016, 36(7): 1596-1601. doi: 10.6023/cjoc201601019

Cu(Ⅱ)/O2体系中α-唑取代酮与2,2,6,6-四甲基哌啶-1-氧化物的α-羟氨基化反应研究
-
关键词:
- 铜催化
- / 氧气
- / α-唑取代酮
- / 2,2,6,6-四甲基哌啶-1-氧化物
- / α-羟氨基化反应
English
Researches on the α-Aminoxylation between α-Azoleketones and 2,2,6,6-Tetramethylpiperidine-1-oxyl with Cu/O2
-
Key words:
- Cu catalyzed
- / dioxygen
- / α-azoleketone
- / 2,2,6,6-tetramethylpiperidine-1-oxyl
- / α-aminoxylation
-
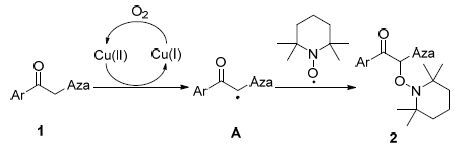
α-唑取代酮是一类重要的有机杂环化合物,在药物化学领域具有较大的应用价值,可作为酶抑制剂、抗癌和抗病毒药物等[1]. 近年,合成并研究多官能团化的α-唑取代酮已经发展成有机化学和药物化学领域的热点之一. 烷氧基胺类化合物在材料化学和有机合成领域具有广泛的用途. 此类化合物可作为自由基反应的引发剂[2]、聚合物稳定剂[3]; 同时,此类化合物也是合成三羰基化合物[4]、α-羟基酮[5]等有机物的中间体. 因此,发展条件温和、简单高效的合成路线具有重要的应用价值和理论意义. 近年,人们发现α-取代酮与2,2,6,6-四甲基哌啶-1-氧化物(TEMPO)的α-羟氨基化反应可以在温和条件下高效的合成烷氧基胺类化合物(Eq. 1). 烷基醛在此类反应中的活性较高,Kazuaki[6]和Jang等[7]以负载的肽或手性氨为催化剂,实现了烷基醛的α-位与TEMPO的羟氨基化反应,得到了非对称的烷氧基胺类化合物. 随后,人们发现α-位被吸电子官能团取代的酮在此类反应表现出更高的活性; 在CAN[8]、铱配合物[9]、二价铜盐[10]催化下,可以快速且高效地实现这类底物的α-羟氨基化反应. 一些课题组将此类反应的底物拓展到α-烷基和酚取代酮; 在大位阻吡啶盐[11]或铜包铁(Cu/Fe)[12]催化下,底物也可以高效发生羟胺化反应. 尽管已有许多关于α-取代酮与TEMPO的羟氨基化反应的报道; 然而α-唑取代酮与TEMPO的α-羟氨基化反应却未曾有人报道. 这里,我们以廉价铜(Ⅱ)盐为催化剂、空 气为氧化剂,在温和条件下实现了α-唑取代酮与TEMPO的羟氨基化反应.
1 结果与讨论
1.1 反应条件优化
以α-苯并咪唑取代苯乙酮(1a)和TEMPO为模板,我们考察了催化剂、溶剂和空气对反应的影响; 结果如表 1所示. 首先,我们以乙腈为溶剂,空气为氧化剂,在室温下考察了一些常见铜催化剂对反应的催化活性. 从表 1可以看出,铜催化剂对反应的影响较大; 一价铜盐(CuI,CuBr,Cu2O)对反应催化效果不好,所得目标产物分离产率全都低于15%(表 1,Entries 1~3); 而二价铜盐对反应的催化效果较好(表 1,Entries 4~6). 这可能是因为二价铜盐具有更好的氧化性,有利于单电子转移过程的发生. 在所尝试的二价铜盐中,Cu(OAc)2•H2O的催化效果最佳,反应气相色谱(GC)产率达到90%(表 1,Entry 7). 在无铜盐存在下,底物没有发生任何反应(表 1,En- try 8). 当我们排除体系的空气,并用氮气(101 kPa)置换后,反应的产率明显下降(表 1,Entry 9). 最后,我们以Cu(OAc)2•H2O为催化剂,空气为氧化剂,就溶剂对反应的影响进行了考察,发现溶剂对反应的影响同样不可忽视. 在我们已经尝试的几种常见的溶剂中,乙腈的效果最佳(表 1,Entries 10~13). 经过以上实验,最终确定该反应体系的最优条件: α-唑取代酮/TEMPO(投料比为1:1.1),Cu(OAc)2•H2O (5 mol%)为催化剂,空气(试管敞口)为氧化 剂,乙腈为溶剂,在室温下反应6 h.

Entry 催化剂 溶剂 产率b/% 1c CuI CH3CN 9 2c CuBr CH3CN 6 3c Cu2O CH3CN 11 4 CuSO4•5H2O CH3CN 88 5 CuCl2•2H2O CH3CN 89 6 CuO CH3CN 71 7 Cu(OAc)2•H2O CH3CN 90 8 — CH3CN 0 9d Cu(OAc)2•H2O CH3CN 35 10 Cu(OAc)2•H2O DMF 36 11 Cu(OAc)2•H2O Toluene 13 12 Cu(OAc)2•H2O THF 24 13 Cu(OAc)2•H2O CH2Cl2 75 aReaction conditions: Cu (5 mol%),1a (0.5 mmol),TEMPO (1.1 equiv.) and solvent (1.5 mL) at room temperature for 6 h under air condition; b Isolated yield; c With Cu(I) salts (30 mol%); d Under N2 atmosphere. 表 1 条件优化a
Table 1. Optimization of reaction conditionsa1.2 反应底物适用范围
在最佳反应条件下,我们对铜催化α-唑取代酮的羟氨基化反应的底物适用性进行了研究. 实验结果如表 2所示,该方法具有较广的底物适用范围. 首先,我们以一系列α-苯并咪唑取代酮为底物,考察了取代基R1对反应的影响. 当R1为对位或间位取代苯基时(如4-CH3,3-OCH3,4-Br,4-F,4-Cl,4-NO2),取代基对反应的影响不大,反应都可以高产率的生成目标产物(表 2,2a~2f). 当R1为邻位取代苯基时(如2-CH3),反应时间需要延长至10 h; 由于底物位阻较大,导致反应的产率下降至79%(表 2,2g). 当R1为无取代苯基时,目标产物的分离产率达到91%(表 2,2h). 当R1为其他芳基时(如1-萘基,2-呋喃基),反应依然具有较高的产率(表 2,2i和2j). 此外,我们还尝试了α-苯并咪唑取代乙酸乙酯在该反应中的活性; 可能是由于酯基不利于自由基的产生,导致反应难以启动,所以该底物没有发生反应(表 2,2k). 接下来,我们以一系列α-唑取代苯乙酮为底物,考察了唑类取代基对反应的影响. 当底物的α-位取代基为咪唑、6-氯嘌呤、取代三唑时,底物具有不错的反应活性,产率介于81%~89%(表 2,2l~2o). 令人遗憾的是,α-(N,N-二乙基胺基)苯乙酮作为底物时,反应并没有发生(表 2,2p).


a Reaction conditions: Cu(OAc)2•H2O (5 mol%),1 (0.5 mmol),TEMPO (0.55 mmol) and CH3CN (1.5 mL) at room temperature for 6 h under air condition; b Isolated yield; c Reaction for 10 h. 表 2 底物适应性考察a,b
Table 2. Scope of the reactiona,b1.3 反应机理分析
根据以往有关α-羟氨基化反应的报道和我们的实验结果,我们推测该反应有可能经历了一个单电子转移过程,其可能的机理如Scheme 1所示. 底物首先被二价铜盐氧化,发生一个单电子转移过程,产生了自由基中间体A和一价铜盐[13]; 随后,TEMPO捕捉该中间体,并发生α-羟氨基化反应. 过程所产生的一价铜盐被氧气氧化为二价铜盐后可以启动下一个催化循环[14]. 一价铜在反应中表现出较低的催化活性,其原因可能是催化剂的还原产物在反应条件下不易被氧气氧化, 难以实现催化循环.
2 结论
本文以廉价二价铜盐为催化剂,以空气为氧化剂,在室温下实现了α-唑取代酮与TEMPO的α-羟氨基化反应. 反应具有催化剂廉价、氧化剂环保、条件温和、效率高等优点. 此外,我们以此方法合成了13个烷氧胺类化合物,为这些化合物的合成提供了新方法.
3 实验部分
3.1 仪器与试剂
美国PERKIN-ELIMER 1730 FT-IR红外光谱仪,KBr压片; 德国Bruker 公司400 MHz DRX-400核磁共振仪,溶剂为CDCl3,内标TMS; 美国Thermo公司TSQ Quantum Ultra质谱仪(ESI); 河南省予华仪器有限公司X-5型显微熔点仪,温度计未校正. 所有试剂均为市售分析纯,未经进一步纯化.
3.2 实验方法
α-苯并咪唑取代苯乙酮[15]、α-咪唑取代苯乙酮、α-(6-氯嘌呤)取代苯乙酮和α-三唑取代苯乙酮[16]的合成方法见参考文献.
α-唑取代酮与TEMPO的α-羟氨基化反应一般步骤: 在装有磁子的15 mL刻度试管中加入α-唑取代酮(0.5 mmol)、TEMPO(0.55 mmol)、Cu(OAc)2•H2O (5 mol%)和乙腈(1.5 mL),然后将试管敞口在空气中室温搅拌反应6 h. 当反应物消失后(用TLC跟踪),将混合物倒入15 mL饱和食盐水中,并用乙酸乙酯(15 mL×3)萃取,合并有机层,用无水硫酸镁干燥,过滤,旋干溶剂得粗产物. 然后以石油醚和乙酸乙酯为洗脱剂(体积比为2:1~5:1),通过硅胶柱层析分离得到目标化合物.
2-(1H-苯并咪唑-1-基)-2-(2,2,6,6-四甲基哌啶-1-基)氧基-1-(4-甲基苯基)乙酮(2a): 透明粘稠流体,产率90%. 1H NMR (400 MHz,CDCl3) δ: 8.17 (s,1H),7.83 (d,J=4.0 Hz,2H),7.73 (d,J=4.0 Hz,1H),7.68 (d,J=4.0 Hz,1H),7.2 (t,J=8.0 Hz,1H),7.26 (t,J=8.0 Hz,1H),7.19 (d,J=4.0 Hz,2H),7.02 (s,1H),2.33 (s,3H),1.52~1.58 (m,3H),1.31~1.39 (m,3H),1.28 (s,3H),1.16 (s,3H),1.05 (s,3H),0.34 (s,3H); 13C NMR (100 MHz,CDCl3) δ: 190.41,145.27,142.28,131.59,129.72,128.62,123.82,122.80,120.36,111.71,89.35,61.41,59.95,40.07,39.87,33.14,32.14,21.67,20.32,20.21,16.98; IR (KBr) ν: 2968,2930,1697,1601,1453,1277,1235 cm-1; MS (ESI) m/z: 406 [M+H]+. Anal. calcd for C25H31N3O2: C 74.04,H 7.71,N 10.36; found C 73.87,H 7.61,N 10.47
2-(1H-苯并咪唑-1-基)-1-(3-甲氧基苯基)-2-[(2,2,6,6-四甲基哌啶-1-基)氧基]乙酮(2b): 无色晶体,产率88%. m.p. 127~129 ℃; 1H NMR (400 MHz,CDCl3) δ: 8.17 (s,1H),7.74 (d,J=4.0 Hz,1H),7.69 (d,J=4.0 Hz,1H),7.50 (d,J=4.0 Hz,1H),7.42 (s,1H),7.25~7.42 (m,4H),7.03~7.06 (m,2H),3.75 (s,3H),1.48~1.60 (m,3H),1.34~1.40 (m,3H),1.29 (s,3H),1.17 (s,3H),1.05 (s,3H),0.34 (s,3H); 13C NMR (100 MHz,CDCl3) δ: 190.64,160.00,143.62,142.31,135.36,129.98,120.40,112.91,111.69,89.42,61.41,59.95,55.41,40.04,39.84,32.15,20.30,20.18,16.96; IR (KBr) ν: 2971,2936,1700,1588,1495,1456,1251 cm-1; MS (ESI) m/z: 422 [M+H]+. Anal. calcd for C25H31N3O3: C 71.23,H 7.41,N 9.97; found C 71.08,H 7.50,N 10.06;2-(1H-苯并咪唑-1-基)-1-(4-溴苯基)-2-[(2,2,6,6-四甲基哌啶-1-基)氧基]乙酮(2c): 透明粘稠流体,产率89%;1H NMR (400 MHz,CDCl3) δ: 8.16 (s,1H),7.79 (d,J=4.0 Hz,2H),7.75 (d,J=4.0 Hz,1H),7.64 (d,J=4.0 Hz,1H),7.53 (d,J=4.0 Hz,2H),7.33 (t,J=8.0 Hz,1H),7.27 (t,J=8.0 Hz,1H),6.98 (s,1H),1.53~1.60 (m,3H),1.31~1.40 (m,3H),1.27 (s,3H),1.15 (s,3H),1.05 (s,3H),0.34 (s,3H); 13C NMR (100 MHz,CDCl3) δ: 190.01,143.68,142.17,132.77,132.42,129.92,129.60,124.02,123.02,120.54,111.47,89.62,61.49,60.03,40.04,39.85,33.12,32.15,20.35,20.24,16.95; IR (KBr) ν: 2975,2933,1697,1585,1453,1271 cm-1; MS (ESI) m/z: 470 [M+H]+. Anal. calcd for C24H28BrN3O2: C 61.28,H 6.00,N 8.93; found C 61.17,H 6.09,N 9.01;
2-(1H-苯并咪唑-1-基)-1-(4-氟苯基)-2-[(2,2,6,6-四甲基哌啶-1-基)氧基]乙酮(2d): 无色晶体,产率86%;m.p. 159~161 ℃; 1H NMR (400 MHz,CDCl3) δ: 8.19 (s,1H),7.99 (t,J=6.0 Hz,2H),7.76 (d,J=4.0 Hz,1H),7.67 (d,J=4.0 Hz,1H),7.33 (t,J=8.0 Hz,1H),7.27 (t,J=8.0 Hz,1H),7.05 (dd,J=16.0,8.0 Hz,3H),1.52~1.59 (m,3H),1.31~1.40 (m,3H) ,1.28 (s,3H),1.16 (s,3H),1.05 (s,3H),0.35 (s,3H); 13C NMR (100 MHz,CDCl3) δ: 189.37,166.13 (d,J=255.0 Hz,1C),142.97 (d,J=149.0 Hz,2C),131.26,130.46,123.96,116.21,111.53,89.58,61.45,60.00,40.03,39.83,32.13,20.33,20.22,16.94; IR (KBr) ν: 2971,2933,1703,1588,1456,1274,1091 cm-1; MS (ESI) m/z: 410 [M+H]+; Anal. calcd for C24H28FN3O2: C 70.39,H 6.89,N 10.26; found C 70.27,H 6.95,N 10.33.
2-(1H-苯并咪唑-1-基)-1-(4-氯苯基)-2-[(2,2,6,6-四甲基哌啶-1-基)氧基]乙酮(2e): 透明粘稠流体,产率87%. 1H NMR (400 MHz,CDCl3) δ: 8.16 (s,1H),7.88 (d,J=4 Hz,2H),7.75 (d,J=4.0 Hz,1H),7.65 (d,J=4.0 Hz,1H),7.36 (d,J=4.0 Hz,2H),7.25~7.32 (m,2H),6.99 (s,1H),1.53~1.60 (m,3H),1.31~1.40 (m,3H),1.28 (s,3H),1.15 (s,3H),1.05 (s,3H),0.35 (s,3H); 13C NMR (100 MHz,CDCl3) δ: 189.90,143.68,142.18,140.79,132.36,129.88,129.42,124.01,123.01,120.53,111.49,89.63,61.48,60.03,40.04,39.85,33.12,32.14,20.34,20.23,16.95; IR (KBr) ν: 2971,1697,1594,1453,1229,1200cm-1; MS (ESI) m/z: 426 [M+H]+. Anal. calcd for C24H28ClN3O2: C 67.67,H 6.63,N 9.87; found C 67.78,H 6.72,N 9.96.
2-(1H-苯并咪唑-1-基)-1-(4-硝基苯基)-2-[(2,2,6,6-四甲基哌啶-1-基)氧基]乙酮(2f): 无色晶体,产率87%. m.p. 110~112 ℃; 1H NMR (400 MHz,CDCl3) δ: 8.23 (d,J=4.0 Hz,2H),8.15 (s,1H),8.06 (d,J=4.0 Hz,2H),7.75 (d,J=4.0 Hz,1H),7.61 (d,J=4.0 Hz,1H),7.35 (t,J=8.0 Hz,1H),7.29 (t,J=8.0 Hz,1H),7.02 (s,1H),1.54~1.61 (m,3H),1.33~1.42 (m,3H), 1.29 (s,3H),1.17 (s,3H),1.06 (s,3H),0.37 (s,3H); 13C NMR (100 MHz,CDCl3) δ: 189.83,150.62,143.71,138.70,129.51,124.28,124.19,123.28,120.70,111.24,90.19,61.62,60.17,40.05,39.85,33.10,32.15,20.35,20.26,16.92; IR (KBr) ν: 2923,1709,1597,1524,1450,1344 cm-1; MS (ESI) m/z: 437 [M+H]+; Anal. calcd for C24H28N4O4: C 66.04,H 6.47,N 12.84; found C 65.94,H 6.58,N 12.95.
2-(1H-苯并咪唑-1-基)-1-(2-甲基苯基)-2-[(2,2,6,6-四甲基哌啶-1-基)氧基]乙酮(2g): 无色晶体,产率79%. m.p. 104~106 ℃; 1H NMR (400 MHz,CDCl3) δ: 7.97 (s,1H),7.71 (d,J=4.0 Hz,1H),7.64 (d,J=4.0 Hz,1H),7.55 (d,J=4.0 Hz,1H),7.26~7.32 (m,3H),7.19 (t,J=7.2 Hz,1H),7.10 (d,J=4 Hz,1H),6.92 (s,1H),2.18 (s,3H),1.52~1.61 (m,3H),1.35~1.39 (m,3H),1.33 (s,3H),1.26 (s,3H),1.04 (s,3H),0.32 (s,3H); 13C NMR (100 MHz,CDCl3) δ: 194.04,143.64,142.49,139.52,134.70,132.39,132.08,127.01,125.67,123.71,122.83,120.35,111.87,90.81,61.44,59.94,40.10,39.94,33.06,32.18,20.47,20.27,20.16,16.98; IR (KBr) ν: 2927,1703,1604,1453,1277 cm-1; MS (ESI) m/z: 406 [M+H]+. Anal. calcd for C25H31N3O2: C 74.04,H 7.71,N 10.36; found C 73.90,H 7.79,N 10.43.
2-(1H-苯并咪唑-1-基)-1-苯基-2-[(2,2,6,6-四甲基哌啶-1-基)氧基]乙酮(2h): 无色晶体,产率91%. m.p. 144~146 ℃; 1H NMR (400 MHz,CDCl3) δ: 8.19 (s,1H),7.94 (d,J=4.0 Hz,2H),7.73 (dd,J=20.0,8.0 Hz,2H),7.54 (t,J=7.4 Hz,1H),7.41 (t,J=8.0 Hz,2H),7.35 (t,J=7.4 Hz,1H),7.28 (t,J=8.0 Hz,1H),7.07 (s,1H),1.55~1.62 (m,3H),1.33~1.45 (m,3H),1.30 (s,3H),1.19 (s,3H),1.06 (s,3H),0.35 (s,3H); 13C NMR (100 MHz,CDCl3) δ: 190.86,143.62,142.31,134.15,134.09,132.47,130.96,129.04,128.47,123.91,122.90,120.40,111.71,89.38,61.45,59.97,40.04,39.84,32.15,20.32,20.20,16.97; IR (KBr) ν: 2930,1700,1492,1271,1229 cm-1; MS (ESI) m/z: 392 [M+H]+. Anal. calcd for C24H29N3O2: C 73.67,H 7.47,N 10.73; found C 73.58,H 7.53,N 10.85.
2-(1H-苯并咪唑-1-基)-1-(2-萘基)-2-[(2,2,6,6-四甲基哌啶-1-基)氧基]乙酮(2i): 无色晶体,产率84%. m.p. 185~187 ℃; 1H NMR(400 MHz,CDCl3) δ: 8.48 (s,1H),8.23 (s,1H),7.96 (d,J=4.0 Hz,1H),7.88 (d,J=4.0 Hz,1H),7.82 (t,J=8.0 Hz,2H),7.74 (t,J=8.0 Hz,2H),7.58 (t,J=8.0 Hz,1H),7.52 (t,J=8.0 Hz,1H),7.35 (t,J=8.0 Hz,1H),7.26 (t,J=8.0 Hz,1H),7.17(s,1H),1.54~1.59 (m,3H),1.39~1.42 (m,2H),1.34~1.36 (m,1H),1.30 (s,3H),1.19 (s,3H),1.08 (s,3H),0.38 (s,3H); 13C NMR (100 MHz,CDCl3) δ: 190.79,135.88,132.29,131.40,130.49,129.73,129.22,129.05,127.80,127.17,123.88,122.86,120.49,111.63,89.64,61.47,60.02,40.09,39.88,33.19,32.22,20.38,20.25,16.99; IR (KBr) ν: 2955,2923,1690,1661,1460,1274 cm-1; MS (ESI) m/z: 442 [M+H]+. Anal. calcd for C28H31N3O2: C 76.16,H 7.08,N 9.52; found C 76.07,H 7.13,N 9.61.
2-(1H-苯并咪唑-1-基)-2-[(2,2,6,6-四甲基哌啶-1-基)氧基]-1-(2-噻吩基)乙酮(2j): 无色晶体,产率83%. m.p. 182~184 ℃; 1H NMR (400 MHz,CDCl3) δ: 8.21 (s,1H),7.83(d,J=2.0 Hz,1H),7.76 (d,J=8.0 Hz,1H),7.67 (d,J=8.0 Hz,1H),7.64 (d,J=2.0 Hz,1H),7.32 (t,J=8.0 Hz,1H),7.27 (t,J=8.0 Hz,1H),7.07(t,J=4.0 Hz,1H),6.78 (s,1H),1.50~1.57 (m,3H),1.33~1.39 (m,3H),1.28 (s,3H),1.19 (s,3H),1.06 (s,3H),0.34 (s,3H); 13C NMR (100 MHz,CDCl3) δ: 183.72,143.70,142.24,140.04,135.66,133.53,132.68,128.58,123.89,122.89,120.46,111.54,90.92,61.51,60.01,40.08,39.88,33.18,32.06,20.44,20.36,16.94; IR (KBr) ν: 2927,1729,1674,1460,1271,1197 cm-1; MS (ESI) m/z: 398 [M+H]+. Anal. calcd for C22H27N3O2S: C 66.47,H 6.85,N 10.57,S 8.06; found C 66.31,H 6.92,N 10.63,S 7.96.
2-(1H-咪唑-1-基)-1-苯基-2-[(2,2,6,6-四甲基哌啶-1-基)氧基]乙酮(2l): 无色晶体,产率88%. m.p. 122~124 ℃; 1H NMR (400 MHz,CDCl3) δ: 8.00 (d,J=4.0 Hz,2H),7.76 (s,1H),7.59 (t,J=8.0 Hz,1H),7.47 (t,J=8.0 Hz,2H),7.09 (s,1H),7.02 (s,1H),6.66 (s,1H),1.45~1.50 (m,4H),1.32~1.38 (m,1H),1.24~1.27 (m,1H),1.18 (s,3H),1.12 (s,3H),1.07 (s,3H),0.58 (s,3H); 13C NMR (100 MHz,CDCl3) δ: 191.41,137.29,134.22,133.94,130.17,129.01,128.89,117.55,90.95,61.31,59.83,40.11,39.80,33.09,31.94,20.44,20.25,16.97; IR (KBr) ν: 2927,1697,1597,1453,1255,1072 cm-1; MS (ESI) m/z: 342 [M+H]+. Anal. calcd for C20H27N3O2: C 70.35,H 7.97,N 12.31; found C 70.21,H 8.03,N 12.41.
2-(6-氯-9H-嘌呤-9-基)-1-苯基-2-[(2,2,6,6-四甲基哌啶-1-基)氧基]乙酮(2m): 无色晶体,产率81%. m.p. 105~107 ℃; 1H NMR (400 MHz,CDCl3) δ: 8.86 (s,1H),8.44 (s,1H),8.15 (d,J=4.0 Hz,2H),7.60 (t,J=6.0 Hz,1H),7.48 (dd,J=16.0,8.0 Hz,3H),1.50~1.59 (m,3H),1.38~1.40 (m,2H),1.31~1.33 (m,1H),1.22 (s,3H),1.11 (s,3H),1.08 (s,3H),0.31 (s,3H); 13C NMR (100 MHz,CDCl3) δ: 191.42,152.48,151.52,151.24,143.37,134.79,133.67,131.03,129.18,128.91,85.76,61.3 5,59.94,39.84,39.70,33.07,32.57,20.19,20.10,16.89; IR (KBr) ν: 2933,1693,1588,1562,1335,1181,1130 cm-1; MS (ESI) m/z: 428 [M+H]+. Anal. calcd for C22H26Cl- N5O2: C 61.75,H 6.12,N 16.37; found C 61.67,H 7.53,N 16.44.
1-苯基-2-(4-苯基-1H-1,2,3-三唑-1-基)-2-[(2,2,6,6-四甲基哌啶-1-基)氧基]乙酮(2n): 无色晶体,产率89%. m.p. 152~154 ℃; 1H NMR (400 MHz,CDCl3) δ: 8.15 (d,J=4.0 Hz,2H),8.02 (s,1H),7.84 (d,J=4.0 Hz,2H),7.61 (t,J=8.0 Hz,1H),7.49 (t,J=8.0 Hz,2H),7.42 (t,J=8.0 Hz,3H),7.34 (t,J=8.0 Hz,1H),1.48~1.57 (m,5H),1.34~1.38 (m,1H),1.24 (s,3H),1.12 (s,3H),1.11 (s,3H),0.57 (s,3H); 13C NMR (100 MHz,CDCl3) δ: 191.00,148.53,133.58,129.23,128.85,125.76,118.08,92.00,61.42,59.94,39.99,39.75,32.13,20.43,20.14,16.98; IR (KBr) ν: 2934,1693,1508,1453,1229 cm-1; MS (ESI) m/z: 419 [M+H]+. Anal. calcd for C25H30N4O2: C 71.74,H 7.23,N 13.39; found C 71.68,H 7.35,N 13.51.
2-(4-环丙基-1H-1,2,3-三唑-1-基)-1-苯基-2-[(2,2,6,6-四甲基哌啶-1-基)氧基]乙酮(2o): 无色粘稠液体,产率85%. 1H NMR (400 MHz,CDCl3) δ: 8.10 (d,J=4.0 Hz,2H),7.59 (t,J=8.0 Hz,1H),7.46 (t,J=8.0 Hz,3H),7.29 (s,1H),1.88~1.95 (m,1H),1.44~1.53 (m,5H),1.31~1.35 (m,1H),1.19(s,3H),1.09 (s,3H),1.06 (s,3H),0.90~0.92 (m,2H),0.77~0.81 (m,2H),0.49 (s,3H); 13C NMR (100 MHz,CDCl3) δ: 191.05,151.21,134.46,133.68,128.20,128.99,118.22,91.87,61.31,59.84,40.0 0,39.71,31.82,20.43,20.12,16.98,7.93,7.88,6.72; IR (KBr) ν: 2936,1699,1683,1558 cm-1; MS (ESI) m/z: 383 [M+H]+. Anal. calcd for C22H30N4O2: C 69.08,H 7.91,N 14.65; found C 69.01,H 7.99,N 14.72.
辅助材料(Supporting Information) 化合物2a~2j和2l~2o的核磁共振氢谱和碳谱. 这些材料可以免费从本刊网站(http://sioc-journal.cn/)上下载.
-
-
[1]
Erdmann, A.; Menon, Y.; Gros, C.; Molinier, N.; Novosad, N.; Samson, A.; Gregoire, J.; Long, C.; Ausseil, F.; Hlby, L.; Arimondo, P. B. Bioorg. Med. Chem. 2015, 23, 5946 (b) Lanier, M.; Sergienko, E.; Simão A. M.; Su, Y.; Chung, T.; Millán, J. L.; Cashman, J. R. Bioorg. Med. Chem. 2010, 18, 573 (c) Salerno, L; Modica, M. N.; Romeo, G.; Pittalà, V.; Siracusa, M. A.; Amato, M. E.; Acquaviva, R.; Giacomo, C. D.; Sorrenti, V. Eur. J. Med. Chem. 2012, 49, 118 (d) Peifer, C.; Bühler, S.; Hauser, D.; Kinkel, K.; Totzke, F.; Schächtele, C.; Laufer, S. Eur. J. Med. Chem. 2009, 44, 1788 (e) Wei, Q.-L.; Zhang, S.-S.; Gao, J.; Li, W.-H.; Xu, L.-Z.; Yu, Z.-G. Bioorg. Med. Chem. 2006, 14, 7146 (f) Pautus, S.; Sehr, P.; Lewis, J.; Fortuné, A.; Wolkerstorfer, A.; Szolar, O.; Guilligay, D.; Lunardi, T.; Décout, J.-L.; Cusack, S. J. Med. Chem. 2013, 56, 8915. 10.1016/j.bmc.2015.06.066
-
[2]
Hawker, C. J.; Bosmann, A. W.; Harth, E. Chem. Rev. 2001, 101, 3661. (b) Benoit, D.; Chaplinski, V.; Braslau, R.; Hawker, C. J. J. Am. Chem. Soc. 1999, 121, 3904.
-
[3]
Sciannamea V.; Jérôme, R.; Detrembleur C. Chem. Rev. 2008, 108, 1104. (b) Calabrese, D. R.; Ditter, D.; Liedel, C.; Blumfield, A.; Zentel, R.; Ober, C. K. ACS Macro. Lett. 2015, 4, 606.
-
[4]
Wang, Z.-L.; An, X.-L.; Ge, L.-S.; Jin, J.-H.; Luo, X.; Deng, W.-P. Tetrahedron 2014, 70, 3788.
-
[5]
Dinca, E.; Hartmann, P.; Smrček, J.; Dix, I.; Jones, P. G.; Jahn, U. Eur. J. Org. Chem. 2012, 4461. (b) Kirchberg, S.; Fröhlich, R.; Studer, A. Angew. Chem., Int. Ed. 2010, 49, 6877. (c) Abeykoon, G. A.; Chatterjee, S.; Chen, J. S. Org. Lett. 2014, 16, 3248.
-
[6]
Akagawa, K.; Fujiwara, T.; Sakamoto, S.; Kudo, K. Chem. Commun. 2010, 46, 8040.
-
[7]
Bui, N.-N.; Ho, X.-H.; Mho, S.-I.; Jang, H.-Y. Eur. J. Org. Chem. 2009, 5309.
-
[8]
Feng, P.; Song, S.; Zhang, L.-H.; Jiao, N. Synlett 2014, 25, 2717.
-
[9]
Koike, T.; Yasu, Y.; Akita, M. Chem. Lett. 2012, 41, 999.
-
[10]
Luo, X.; Wang, Z.-L.; Jin, J.-H.; An, X.-L.; Shen, Z.; Deng, W.-P. Tetrahedron 2014, 70, 8226.
-
[11]
Li, Y.; Pouliot, M.; Vogler, T.; Renaud, P.; Studer, A. Org. Lett. 2012, 14, 4474.
-
[12]
Xie, Y.-X.; Song, R.-J.; Liu, Y.; Liu, Y.-Y.; Xiang, J.-N.; Li, J.-H. Adv. Synth. Catal. 2013, 355, 3387.
-
[13]
Selected reviews on reaction via Cu-catalyzed single electron transfer: (a) McCann, S. D.; Stahl, S. S. Acc. Chem. Res. 2015, 48, 1756. (b) Yu, H.; Su, S.; Chi, Z.; Dang, Z. -M. Chin. J. Org. Chem. 2013, 33, 1628 (in Chinese). (于海珠, 苏圣钦, 张弛, 党智敏, 有机化学, 2013, 33, 1628.) 10.1021/acs.accounts.5b00060
-
[14]
Selected reviews on Cu-catalyzed oxidation with molecular oxygen: (a) Punniyamurthy, T.; Velusamy, S.; Iqbal J. Chem. Rev. 2005, 105, 2329. (b) Campbell, A. N.; Stahl, S. S. Acc. Chem. Res. 2012, 45, 851. (c) Allen, S. E.; Walvoord, R. R.; Padilla-Salinas, R.; Kozlowski, M. C. Chem. Rev. 2013, 113, 6234.Some examples on Cu-catalyzed oxidation with molecular oxygen: (d) Gao, H.; Wang, H.; Huang, Z.; Yao, L.; Peng, J.; Chen, C. Chin. J. Org. Chem. 2015, 35, 1707 (in Chinese). (高翯, 王瀚旸, 黄章杰, 姚丽萍, 彭进松, 陈春霞, 有机化学, 2015, 35, 1707.) (e) Li, J.; Zhang, Z.; Li, C.; Luo, W.; Yang, S. Chin. J. Org. Chem. 2015, 35, 2199 (in Chinese). (李建晓, 张振明, 李春生, 罗维, 杨少容, 有机化学, 2015, 35, 2199.)
-
[15]
Tsai, A. S.; Wilson, R. M.; Harada, H.; Berqman, R. G.; Ellman, J. A. Chem. Commun. 2009, 3910 (b) Zhang, Y.; Zhang, Y.; Xiao, J.; Peng, Z.; Dong, W.; An, D. Eur. J. Org. Chem. 2015, 35, 7806.
-
[16]
Kumar, D.; Reddy, V. B.; Kumar, A.; Mandal, D.; Tiwari, R.; Parang, K. Bioorg. Med. Chem. Lett. 2011, 21, 449. 10.1016/j.bmcl.2010.10.121
-
[1]
-
表 1 条件优化a
Table 1. Optimization of reaction conditionsa

Entry 催化剂 溶剂 产率b/% 1c CuI CH3CN 9 2c CuBr CH3CN 6 3c Cu2O CH3CN 11 4 CuSO4•5H2O CH3CN 88 5 CuCl2•2H2O CH3CN 89 6 CuO CH3CN 71 7 Cu(OAc)2•H2O CH3CN 90 8 — CH3CN 0 9d Cu(OAc)2•H2O CH3CN 35 10 Cu(OAc)2•H2O DMF 36 11 Cu(OAc)2•H2O Toluene 13 12 Cu(OAc)2•H2O THF 24 13 Cu(OAc)2•H2O CH2Cl2 75 aReaction conditions: Cu (5 mol%),1a (0.5 mmol),TEMPO (1.1 equiv.) and solvent (1.5 mL) at room temperature for 6 h under air condition; b Isolated yield; c With Cu(I) salts (30 mol%); d Under N2 atmosphere. 表 2 底物适应性考察a,b
Table 2. Scope of the reactiona,b


a Reaction conditions: Cu(OAc)2•H2O (5 mol%),1 (0.5 mmol),TEMPO (0.55 mmol) and CH3CN (1.5 mL) at room temperature for 6 h under air condition; b Isolated yield; c Reaction for 10 h. -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 0
- 文章访问数: 1461
- HTML全文浏览量: 140


 下载:
下载:
 下载:
下载:

