Citation:
. CCS Chemistry:金黄色葡萄球菌醛基脱氢酶是如何识别特异性底物的?
[J]. CCS Chemistry,
;2020, 2(0): 946-954.
doi:
10.31635/ccschem.020.202000219

-
中山大学夏炜、香港大学孙红哲课题组通过解析金黄色葡萄球菌醛基脱氢酶纯蛋白以及复合物晶体结构,发现其特有的“C-helix”结构域在结合特异性底物过程中发生的构象变化,提出了金黄色葡萄球菌醛基脱氢酶识别特异性底物的分子门控机制,并通过一系列生化实验辅助验证。
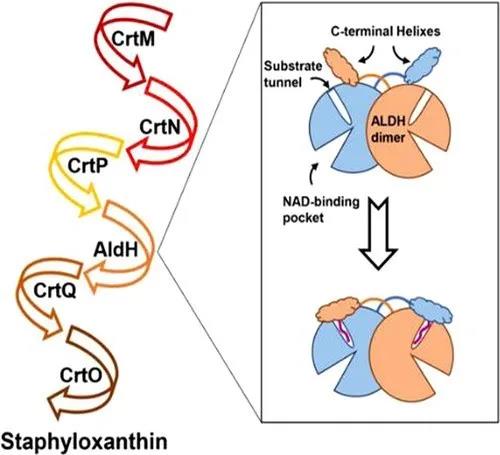
金黄色葡萄球菌是一种常见人类病原菌,可引起一系列感染性疾病,由于抗生素的滥用,产生了许多耐药性的金黄色葡萄球菌,而相对于开发新的抗生素,发展新的抗感染疗法对于对抗病毒感染更为有效。超过90%的金黄色葡萄球菌临床分离株会产生一种金黄色的含有30个碳(C30)的长链类胡萝卜素分子,称为葡萄球菌黄素,该色素可作为抗氧化剂提高细菌对于活性氧的耐受能力。因此,阻断其生物合成途径是一项重要的工作。
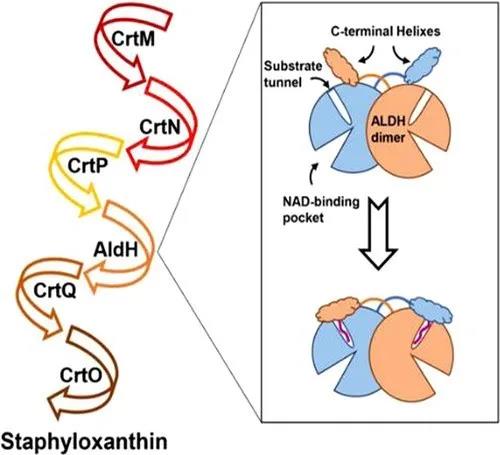
葡萄球菌黄素的生物合成路径需要一系列催化酶的参与(图1)。其中,醛基脱氢酶(SaAldH)是最近被发现的参与色素合成的酶类,其功能是催化长链不饱和醛4,4’- diaponeurosporen-4-al生成对应的羧酸。近期,中山大学夏炜课题组以及香港大学孙红哲课题组报道了SaAldH纯蛋白以及其与特异性底物复合物的晶体结构,在分子层面揭示了SaAldH识别特异性底物——多不饱和长碳链脂肪醛的门控机制。
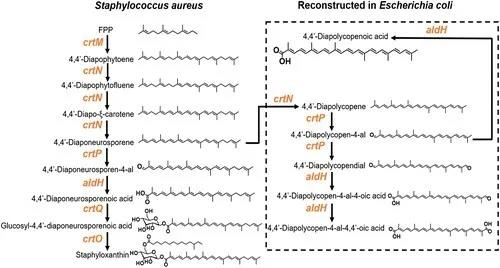
图1 葡萄球菌黄素合成通路示意图
从纯蛋白结构中,作者发现SaAldH二聚体除了保守的催化结构域、NAD结合结构域以及桥连结构域以外,在C端还具有独特的“C-helix”结构,类似于人源脂肪醛脱氢酶(FALDH)中的“gatekeeper helix”。将纯蛋白和复合物晶体结构对比后,发现“C-helix”在结合特异性底物之后发生了明显的构象变化(图2),朝着底物通道入口的方向转动了10.6°的夹角,从而使得氨基酸残基F456和F457的侧链插入到底物通道入口处,破坏了纯蛋白状态下F456、F457以及Y92形成的T型π-π堆叠相互作用。此外“C-helix”之前的loop区域的氨基酸V441和H442也向着底物通道入口的方向靠近,从而实现了底物口袋的闭合。因此作者们提出“C-helix”充当了重要的门控作用,在纯蛋白状态下,酶活口袋处于“开合”状态,允许底物的进入,而当酶结合了特异性底物之后,“C-helix”发生构象变化,在通道入口处形成位阻,锁住底物,使得酶活口袋处于“闭合”状态。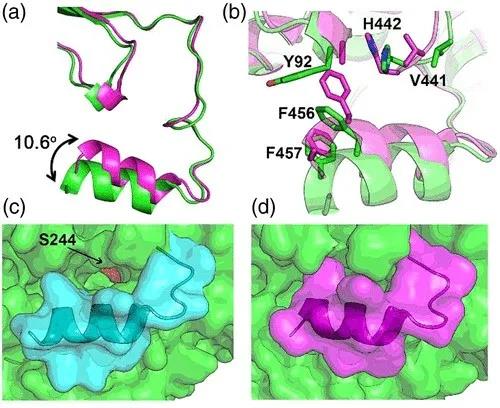 图2 SaAldH的“open-closed”构象变化
图2 SaAldH的“open-closed”构象变化通过对SaAldH和特异性底物之间的相互作用进行分析,作者们发现特异性底物与SaAldH底物结合通道中的氨基酸残基形成广泛的疏水相互作用。另外,酶序列上Y116与底物的2-甲基形成π–σ相互作用,F457与底物末端的两个甲基形成疏水相互作用,暗示着“C-helix”同时也参与了对特异性底物的识别(图3)。
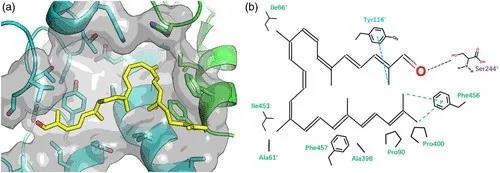
图3 SaAldH与其特异性底物相互作用分析最后,作者们通过体外酶活实验、过氧化氢耐受实验以及巨噬细胞吞噬实验对关键氨基酸位点进行验证,证实结构中看到的关键性位点确实参与了特异性底物的识别(图4)。
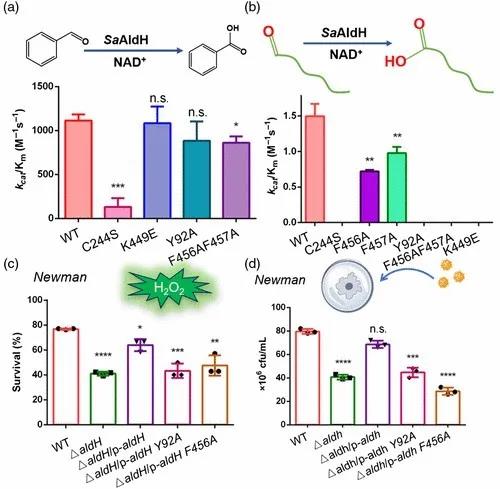
图4 关键氨基酸参与特异性底物识别的生化实验验证此项研究得到了国家自然科学基金、香港研究资助局、中国教育部以及中央高校基础研究经费的资助。该工作以research article的形式发表在CCS Chemistry,并在官网“Just Published”栏目上线。
文章详情:
Structural Insight into the Substrate Gating Mechanism by Staphylococcus aureus Aldehyde Dehydrogenase
Xuan Tao , Zhemin Zhang , Xiao Zhang , Hongyan Li, Hongzhe Sun *, Zong-Wan Mao& Wei Xia *
Citation:CCS Chem. 2020, 2, 946–954
Link:https://doi.org/10.31635/ccschem.020.202000219
图2 SaAldH的“open-closed”构象变化 -

-

-
-
[1]
Cailiang Yue , Nan Sun , Yixing Qiu , Linlin Zhu , Zhiling Du , Fuqiang Liu . A direct Z-scheme 0D α-Fe2O3/TiO2 heterojunction for enhanced photo-Fenton activity with low H2O2 consumption. Chinese Chemical Letters, 2024, 35(12): 109698-. doi: 10.1016/j.cclet.2024.109698
-
[2]
Jiaxing Cai , Wendi Xu , Haoqiang Chi , Qian Liu , Wa Gao , Li Shi , Jingxiang Low , Zhigang Zou , Yong Zhou . Highly Efficient InOOH/ZnIn2S4 Hollow Sphere S-Scheme Heterojunction with 0D/2D Interface for Enhancing Photocatalytic CO2 Conversion. Acta Physico-Chimica Sinica, 2024, 40(11): 2407002-0. doi: 10.3866/PKU.WHXB202407002
-
[3]
Shunyu Wang , Yanan Zhu , Yang Zhao , Wanli Nie , Hong Meng . Steric effects and electronic manipulation of multiple donors on S0/S1 transition of Dn-A emitters. Chinese Chemical Letters, 2025, 36(4): 110555-. doi: 10.1016/j.cclet.2024.110555
-
[4]
Junchen Peng , Zhongyuan Guo , Dandan Dong , Yusheng Lu , Bao Wang , Fangjie Lu , Chaofeng Huang , Bin Dai . Cu0/Cuδ+ site construction and its catalytic role in acetylene hydrochlorination. Chinese Chemical Letters, 2025, 36(8): 111208-. doi: 10.1016/j.cclet.2025.111208
-
[5]
Jiayuan Liang , Xin Mi , Songhao Guo , Hui Luo , Kejun Bu , Tonghuan Fu , Menglin Duan , Yang Wang , Qingyang Hu , Rengen Xiong , Peng Qin , Fuqiang Huang , Xujie Lü . Pressure-induced emission in 0D metal halide (EATMP)SbBr5 by regulating exciton-phonon coupling. Chinese Journal of Structural Chemistry, 2024, 43(7): 100333-100333. doi: 10.1016/j.cjsc.2024.100333
-
[6]
Yunqing Zhu , Kaiyue Wen , Xuequan Wan , Gaigai Dong , Junfeng Niu . High efficiency conversion of low concentration nitrate boosted with amorphous Cu0 nanorods prepared via in-situ reconstruction. Chinese Chemical Letters, 2025, 36(6): 110399-. doi: 10.1016/j.cclet.2024.110399
-
[7]
.
CCS Chemistry | 超分子活化底物为自由基促进高效选择性光催化氧化
. CCS Chemistry, 2025, 7(10.31635/ccschem.025.202405229): -. -
[8]
Yong Shu , Xing Chen , Sai Duan , Rongzhen Liao . How to Determine the Equilibrium Bond Distance of Homonuclear Diatomic Molecules: A Case Study of H2. University Chemistry, 2024, 39(7): 386-393. doi: 10.3866/PKU.DXHX202310102
-
[9]
Zhiyuan TONG , Ziyuan LI , Ke ZHANG . Three-dimensional porous collector based on Cu-Li6.4La3Zr1.4Ta0.6O12 composite layer for the construction of stable lithium metal anode. Chinese Journal of Inorganic Chemistry, 2025, 41(3): 499-508. doi: 10.11862/CJIC.20240238
-
[10]
Zhenjie Yang , Chenyang Hu , Xuan Pang , Xuesi Chen . Sequence design in terpolymerization of ε-caprolactone, CO2 and cyclohexane oxide: Random ester-carbonate distributions lead to large-span tunability. Chinese Chemical Letters, 2024, 35(5): 109340-. doi: 10.1016/j.cclet.2023.109340
-
[11]
Lei Feng , Ze-Min Zhu , Ying Yang , Zongbin He , Jiafeng Zou , Man-Bo Li , Yan Zhao , Zhikun Wu . Long-Pursued Structure of Au23(S-Adm)16 and the Unexpected Doping Effects. Acta Physico-Chimica Sinica, 2024, 40(5): 2305029-0. doi: 10.3866/PKU.WHXB202305029
-
[12]
Peipei Sun , Jinyuan Zhang , Yanhua Song , Zhao Mo , Zhigang Chen , Hui Xu . Built-in Electric Fields Enhancing Photocarrier Separation and H2 Evolution. Acta Physico-Chimica Sinica, 2024, 40(11): 2311001-0. doi: 10.3866/PKU.WHXB202311001
-
[13]
Lu Huang , Jiang Wang , Hong Jiang , Lanfang Chen , Huanwen Chen . On-line determination of selenium compounds in tea infusion by extractive electrospray ionization mass spectrometry combined with a heating reaction device. Chinese Chemical Letters, 2025, 36(1): 109896-. doi: 10.1016/j.cclet.2024.109896
-
[14]
Chaochao Wei , Ru Wang , Zhongkai Wu , Qiyue Luo , Ziling Jiang , Liang Ming , Jie Yang , Liping Wang , Chuang Yu . Revealing the size effect of FeS2 on solid-state battery performances at different operating temperatures. Chinese Chemical Letters, 2024, 35(6): 108717-. doi: 10.1016/j.cclet.2023.108717
-
[15]
Chang LIU , Chao ZHANG , Tongbu LU . Small-size Au nanoparticles anchored on pyrenyl-graphdiyne for N2 electroreduction. Chinese Journal of Inorganic Chemistry, 2025, 41(1): 174-182. doi: 10.11862/CJIC.20240305
-
[16]
Baohua LÜ , Yuzhen LI . Anisotropic photoresponse of two-dimensional layered α-In2Se3(2H) ferroelectric materials. Chinese Journal of Inorganic Chemistry, 2024, 40(10): 1911-1918. doi: 10.11862/CJIC.20240105
-
[17]
Ying Zhao , Yin-Hang Chai , Tian Chen , Jie Zheng , Ting-Ting Li , Francisco Aznarez , Li-Long Dang , Lu-Fang Ma . Size-controlled synthesis and near-infrared photothermal response of Cp* Rh-based metalla[2]catenanes and rectangular metallamacrocycles. Chinese Chemical Letters, 2024, 35(6): 109298-. doi: 10.1016/j.cclet.2023.109298
-
[18]
Zhenzhen Zhao , Meichen Jiao , Jiejie Ling , Han Jiang , Yan Gao , Hao Xu , Hai-Qing Li , Jingang Jiang , Peng Wu , Le Xu . Toward the microporous zeolite family with tunable large-medium cage and pore opening. Chinese Journal of Structural Chemistry, 2024, 43(9): 100336-100336. doi: 10.1016/j.cjsc.2024.100336
-
[19]
Zhenqiang Guo , Huicong Yang , Qian Wei , Shengjun Xu , Guangjian Hu , Shuo Bai , Feng Li . Dual-additives enable stable electrode-electrolyte interfaces for long life Li-SPAN batteries. Chinese Chemical Letters, 2024, 35(5): 108622-. doi: 10.1016/j.cclet.2023.108622
-
[20]
Chao Ma , Peng Guo , Zhongmin Liu . DNL-16: A new zeolitic layered silicate unraveled by three-dimensional electron diffraction. Chinese Journal of Structural Chemistry, 2024, 43(4): 100235-100235. doi: 10.1016/j.cjsc.2024.100235
-
[1]
Metrics
- PDF Downloads(0)
- Abstract views(21119)
- HTML views(1007)

 Login In
Login In




 DownLoad:
DownLoad: