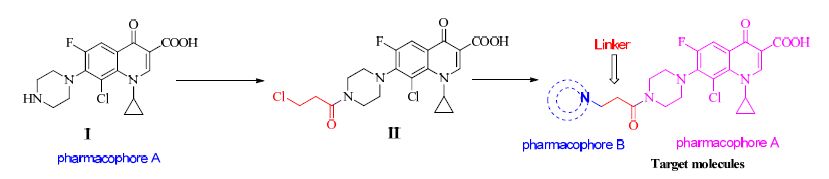
 Figure 1.
Design of intermediate compound II and the target molecules
Figure 1.
Design of intermediate compound II and the target molecules

Synthesis and Characterization of 8-Chloro-7-(4-(3-chloropropanoyl)piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid①
English
Synthesis and Characterization of 8-Chloro-7-(4-(3-chloropropanoyl)piperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylic Acid①
-
Key words:
- fluoroquionolone
- / X-ray diffraction
- / thermal stability
- / photoluminescent
- / antibacterial activity
-
1 INTRODUCTION
Supramolecular chemistry and crystal engineering have emerged into the pharmaceutical field with its importance to understand and predict intermolecular interactions for ready to design crystallized drugs[1-4]. Nowadays, there are two main strategies for the design of crystallized drugs. One is pharmaceutical cocrystals[1, 2, 5, 6] and another is metal-drug complexes[7, 8], which are all widely used in the pharmaceutical field. Fluoroquionolone (FQ), one kind of the most attractive antibacterial agents[9, 10], is generally characterized by a broad antimicrobial spectrum, good bioavailability and tissue penetration, low cardiac toxicity, prolonged serum half life, enhanced tolerability, improved safety and pharmacokinetics properties[11-13]. Although the mechanism of the action of FQs is not completely understood nowadays, two main elucidations have been proposed: the functional targets of FQ antibiotics are DNA-gyrase (topoisomerase II and IV)[14-16], which act by promoting cleavage of bacterial DNA[17]; another one relates the interaction of FQs with DNA via a metal complex intermediate[8]. These FQ drugs, benefiting from continuous improvement and optimization of their structures, have been developed from Nalidixic acid as the representative of the first-generation products[13, 18] to Gatifloxacin on behalf of the fourthgeneration ones[19, 20].
Currently, a lot of FQ supramolecules are reported in literatures[21-25], some cocrystals and salts of FQs are also documented[1, 6]. However, the crystal structures and properties of FQ derivatives are recorded scarely[26]. Even there are 17 molecules containing a piperazine ring in the 28 marketed FQ drugs, compound I (8-chloro-1-cyclopropyl-6- fluoro-4-oxo-7-(piperazin-1-yl)-1, 4-dihydroquinoline- 3-carboxylic acid) is not listed. To develop new antibacterial agents with stronger activity and lower toxicity, the combination of antibacterial FQs with other pharmacophores or drug fragments is a very common strategy. Adopting this design idea, compound II and the target molecules were designed by us (Scheme 1), and then it was prepared in satisfactory yield. What astonished us is that compound II appeared from the solvent with complete single crystal confirmed subsequently by single-crystal X-ray diffraction. For this interesting compound II, other multidimensional characterizations were performed. These initial results are reported in this paper.
2 EXPERIMENTAL
2.1 Instruments and reagents
XRPD data were recorded on an XD-3 diffractometer using CuKa (λ = 1.5406 Å) radiation; TG analysis was performed on a NETZSCH STA 449C instrument in flowing N2 at a heating rate of 10 ℃ min-1; An F-2700 fluorescence spectrophotometer (Hitachi Ltd., Tokyo, Japan) was used to record the fluorescence spectra and measure the fluorescence intensity; A UV-Vis 2450 spectrophotometer (Shimadzu, Japan) was used to record the UV/Vis absorption spectra in the wavelength range of 200~ 800 nm; Nuclear Magnetic Resonance spectrometer (Bruker AVANCE III 600 MHz, 600 MHz for 1H NMR, 151 MHz for 13CNMR, Bruker Baiesibin, Switzerland); Precise Micro Melting Point apparatus (Version X-6, Beijing Fukan Instrument Corporation, Ltd, temperature not rectified, Beijing, China); Solution quantum yield was measured using a Hamamatsu absolute PL quantum yield spectrometer C11347 Quantaurus-QY. Clinafloxacin, Norfloxacin, 8-chloro-1-cyclopropyl-6-fluoro-4-oxo-7- (piperazin-1-yl)-1, 4-dihydroquinoline-3-carbo-xylic acid (95%, Zhengzhou Keer Tai Biochemical Technology Co., Ltd., Zhengzhou, China). All reagents were commercially available and used without further purification.
2.2 Synthesis and characterization of compound II
Compound I (8-chloro-1-cyclopropyl-6-fluoro-4- oxo-7-(piperazin-1-yl)-1, 4-dihydroquinoline-3-carboxylic acid) (18.289 g, 50 mmol), DCM (80 mL) and K2CO3 (20.7 g, 150 mmol) were added to a 500 mL round-bottomed flask, and the resulting mixture was stirred at room temperature for 10 min. 3-Chlo-ropropanoyl chloride (15.871 g, 125 mmol) in DCM (20 mL) was slowly added dropwise under an ice bath. The reaction mixture was stirred continuously under an ice bath. After completion (monitored by TLC, CHCl3/CH3OH, 30/1, V/V), yellow-green turbid solution was obtained. Water (15 mL) was added, and 1 N HCl solution was used to adjust the reaction mixture to pH 6, and extracted with DCM (3 × 20 mL). The combined organic layers, dried over anhydrous Na2SO4 and concentrated by distillation under reduced pressure, give yellow solid. The obtained crude product was scattered in ether (20 mL) with stirring at room temperature, filtrated and then purified by silica gel column chromatography eluting with petroleum ether/DCM (1/20→1/5→1/1, V/V) to give compound II (16.81 g, 74%) as yellow solid. m.p.: 214~216 ℃. 1H NMR (600MHz, CDCl3): δ = 14.26 (s, 1H, COOH), 8.92 (s, 1H, C=CH), 8.06 (d, 1H, J = 11.4 Hz, PhH), 5.30 (m, 2H, CH2Cl), 4.36 (m, 1H, cyclopropane- CH), 3.88 (t, 2H, J = 7.2 Hz, piperazine-CH2), 3.69 (m, 2H, piperazine-CH2), 3.41 (m, 2H, piperazine-CH2), 3.36 (m, 2H, piperazine-CH2), 2.88 (t, 2H, J = 7.2 Hz, COCH2), 1.34~1.31 (m, 2H, cyclopropane-CH2), 0.99 ~ 0.97 (m, 2H, cyclopropane-CH2); 13C NMR (151MHz, CDCl3): δ = 176.75 (quionalone-C=O), 168.42 (chloropro panoyl-C=O), 165.74 (COOH), 157.27 (quionolone- C), 155.59 (PhC), 152.11 (PhC), 143.76 (PhC), 137.85 (PhC), 124.36 (PhC), 120.54 (PhC), 111.90 (quionolone-C), 51.10 (cyclopropane-C), 46.23 (C-Cl), 41.31 (2×piperazine-C), 39.85 (2×piperazine- C), 36.08 (C*C=O), 11.44 (2×cyclopropane-C). HR MS: C20H20Cl2FN3O4 [M+H]+ 456.0888, found 456.0894.
2.3 Crystal data and structure determination
Crystals suitable for X-ray analysis were grown from a DCM solution of compound II by slowly evaporating at room temperature. After about a week, large bright yellow and elongated rhombic crystals are generated. A yellow crystal of compound II having approximate dimensions of 0.20mm × 0.18mm × 0.15mm was mounted on the top of a glass fiber. The unit cell determination and data collection were performed with a CuKα radiation (λ = 1.54184 Å) on a Xcalibur, Eos, Gemini diffractometer equipped with a φ-ω scan mode in the range of 4.48≤θ≤67.07º (-9≤h≤6, -12≤k≤ 12, -19≤l≤18) at 291.15 K. A total of 9024 reflections were collected, of which 4236 were independent (Rint = 0.0217) and 3688 were observed with I > 2σ(I). Absorption corrections were applied using the multiscan technique. Using Olex2[27], the structure was solved with SHELXS[28] structure solution program using the direct methods and refined with the SHELXL[28] refinement package using least-squares minimisation. All non-hydrogen atoms were refined anisotropically. The organic hydrogen atoms attached to carbon atoms were generated geometrically. The disordered C atoms (C(21), C(21)A)/(Cl(3), Cl(3)A)/(Cl(4), Cl(4)A) of compound II were refined using C or Cl atoms split over two sites, with a total occupancy of 1. The final cycle of refinement including 369 variable parameters was converged to R = 0.0534 and wR = 0.1447 (w = 1/[σ2(Fo 2) + (0.0708P)2 + 0.7454P], where P = (Fo 2 + 2Fc 2)/3), S = 1.051, (Δ/σ)max = 0.001, (Δρ)max = 0.46 and (Δρ)min = -0.530 e/Å3.
3 RESULTS AND DISCUSSION
3.1 Description of the crystal structure
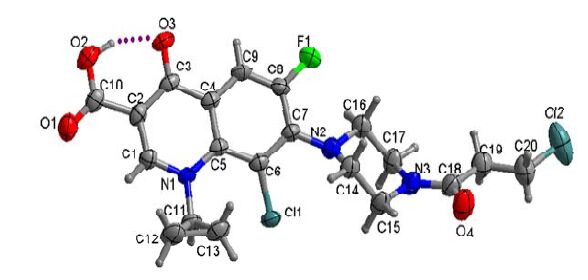
Compound II forms triclinic crystals (P, Z = 2). Selected bond lengths and bond angles are in the normal ranges found in related structures[29]. Bond lengths and bond angles for compound II are listed in Tables 1 and 2. Compound II anhydrate crystallizes with one molecule of compound II and one molecule of CH2Cl2 in the asymmetric unit. The carboxylic acid group is almost coplanar with the FQ moiety (torsion angle O(2)-C(10)-C(2)-C(1) = 175.4(2)°) and participates in intramolecular O-H···O (O-H = 0.9(4) Å, H···O = 1.73(5) Å, O···O = 2.554(3) Å, O-H···O =152(4)°) hydrogen bonding with the carbonyl oxygen atom of the FQ moiety (Fig. 1). The C-O and C=O bond distances of the carboxylic acid group of compound II molecules are 1.325 and 1.207 Å, respectively. The piperazinyl ring is in the usual chair conformation, connected with the quinolone moiety, with torsion angles C(16)-N(2)-C(7)-C(6) to be 150.1(3)° and C(14)- N(2)-C(7)-C(8) of 115.6(3)°. The bond angle C(14)-N(2)-C(16) of piperazinyl ring is 111.5(2)°. The cyclopropyl ring is connected to the quinolone moiety at N atom with the following torsion angles: C(11)-N(1)-C(1)-C(2) 169.3(2)°, C(1)-N(1)-C(11)- C(12)-44.8(4)° and N(1)-C(11)-C(13)-C(12) 107.5(3)°. Moreover, the 3-chloropropanoyl group as a linker is connected to the piperazinyl ring at N atom with the torsion angle C(17)-N(3)-C(18)-O(4) of 179.9(3)°. The investigation of the crystal structure reveals π···π stacking interactions between the adjacent molecules.
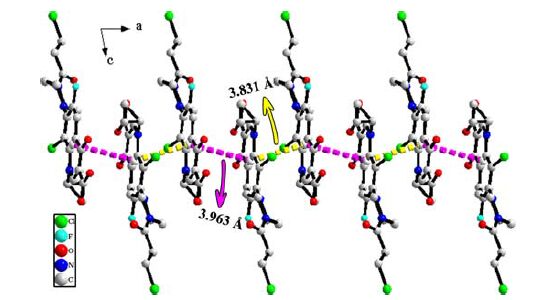
Bond Dist. Cl(1)–C(6) 1.735(2) Cl(2)–C(20) 1.777(4) Cl(3)–C(21) 1.775(10) Cl(4)–C(21) 1.756(10) F(1)–C(8) 1.348(3) O(1)–C(10) 1.207(4) O(2)–C(10) 1.325(4) O(3)–C(3) 1.261(3) O(4)–C(18) 1.227(4) N(1)–C(1) 1.348(3) N(1)–C(5) 1.406(3) N(1)–C(11) 1.469(3) N(2)–C(7) 1.389(3) N(2)–C(14) 1.462(3) N(2)–C(16) 1.467(3) N(3)–C(15) 1.465(4) N(3)–C(17) 1.466(4) N(3)–C(18) 1.346(4) C(1)–C(2) 1.364(4) C(2)–C(3) 1.423(4) C(2)–C(10) 1.487(4) C(3)–C(4) 1.456(4) C(4)–C(5) 1.414(3) C(4)–C(9) 1.400(4) C(5)–C(6) 1.411(3) C(6)–C(7) 1.406(4) C(7)–C(8) 1.402(3) C(8)–C(9) 1.351(4) C(11)–C(12) 1.492(4) C(11)–C(13) 1.478(4) C(12)–C(13) 1.484(5) C(14)–C(15) 1.508(4) C(16)–C(17) 1.513(4) C(18)–C(19) 1.515(4) C(19)–C(20) 1.496(5) C(21)A–Cl(4)A 1.817(15) C(21)A–Cl(3)A 1.751(14) Table 1. Bond Length s (Å) for Compound IIAngle (°) C(1)–N(1)–C(5) 119.4(2) C(1)–N(1)–C(11) 115.4(2) C(5)–N(1)–C(11) 125.0(2) C(7)–N(2)–C(14) 117.4(2) C(7)–N(2)–C(16) 122.4(2) C(14)–N(2)–C(16) 111.5(2) C(15)–N(3)–C(17) 112.9(2) C(18)–N(3)–C(15) 119.9(3) C(18)–N(3)–C(17) 127.2(3) N(1)–C(1)–C(2) 125.2(2) C(1)–C(2)–C(3) 119.2(2) C(1)–C(2)–C(10) 118.4(3) C(3)–C(2)–C(10) 122.4(2) O(3)–C(3)–C(2) 122.9(2) O(3)–C(3)–C(4) 121.0(2) C(2)–C(3)–C(4) 116.1(2) C(5)–C(4)–C(3) 121.7(2) C(9)–C(4)–C(3) 118.0(2) C(9)–C(4)–C(5) 120.3(2) N(1)–C(5)–C(4) 118.0(2) N(1)–C(5)–C(6) 124.6(2) C(6)–C(5)–C(4) 117.4(2) C(5)–C(6)–Cl(1) 122.41(19) C(7)–C(6)–Cl(1) 115.18(18) C(7)–C(6)–C(5) 122.2(2) N(2)–C(7)–C(6) 119.7(2) N(2)–C(7)–C(8) 123.7(2) C(8)–C(7)–C(6) 116.5(2) F(1)–C(8)–C(7) 118.4(2) F(1)–C(8)–C(9) 118.4(2) C(9)–C(8)–C(7) 123.1(2) C(8)–C(9)–C(4) 119.9(2) O(1)–C(10)–O(2) 121.8(3) O(1)–C(10)–C(2) 123.2(3) O(2)–C(10)–C(2) 115.0(3) N(1)–C(11)–C(12) 118.1(2) N(1)–C(11)–C(13) 119.3(2) C(13)–C(11)–C(12) 59.9(2) C(13)–C(12)–C(11) 59.6(2) C(11)–C(13)–C(12) 60.5(2) N(2)–C(14)–C(15) 109.3(2) N(3)–C(15)–C(14) 109.8(2) N(2)–C(16)–C(17) 108.8(2) N(3)–C(17)–C(16) 110.7(2) O(4)–C(18)–N(3) 121.7(3) O(4)–C(18)–C(19) 120.1(3) N(3)–C(18)–C(19) 118.1(3) C(20)–C(19)–C(18) 110.7(3) C(19)–C(20)–Cl(2) 111.3(3) Cl(4)–C(21)–Cl(3) 112.5(5) Cl(3)A–C(21)A–Cl(4)A 110.4(9) Table 2. Bond Angles (Å) for Compound IIAs shown in Fig. 2, the dihedral angle is 67.68° between the cyclopropane ring and fluoroquionolone moiety. There exist two different intermolecular π-π stacking interactions, which have two different distances between ring centroids (Cg(1) is the centroid of the ring (N(1), C(1), C(2), C(3), C(4), C(5), C(6), C(7), C(8), C(9)): Cg(1)···Cg(1) = 3.831 Å (yellow dashes) or Cg(1)···Cg(1) = 3.963 Å (purple dashes)). The fluoroquionolone moieties of two molecules are almost parallel to each other with the dihedral angles of 3.23º and 4.17º, and their face-to-face distances are 3.36 and 3.56 Å, respectively. Therefore, the molecules are further connected by these π···π stacking interactions to form an infinite 1D chain. There is no doubt that such π···π stacking interactions can stabilize the whole framework of compound II.
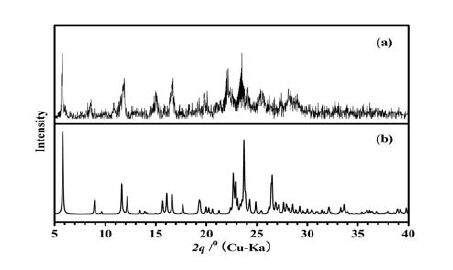
3.2 X-ray powder diffraction
X-ray powder diffraction pattern for compound II was recorded at room temperature (Fig. 3). The peak positions of simulated and experimental patterns are in good agreement with each other, demonstrating the phase purity of the product.
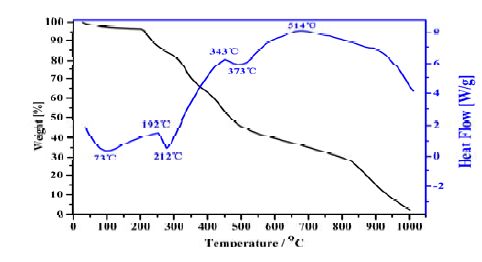
3.3 Thermal stability analyses
The result of thermal gravimetric analysis (TG) of compound II is shown in Fig. 4. The first weight loss is 3.47% in the temperature range of 27~175 ℃, which corresponds to the loss of non-coordinated water molecules (calcd. 3.21%), and then the sample keeps relatively stable in the temperature range of 175~212 ℃; the second weight loss is 15.87% at 212~311 ℃, which corresponds to the release of CH2Cl2 molecules (calcd. 15.18%); the third step is 35.87% from 311 to 514 ℃, and the last step is 13.52% in the temperature range of 514~773 ℃. The whole weight loss is 68.72%, and the endothermic peak at 73, 212 and 373 ℃, the exothermic peaks at 192, 343 and 514 ℃ in the DTA curve of compound II, are all related to the release of non-coordinated water molecules, CH2Cl2 molecules and compound II.
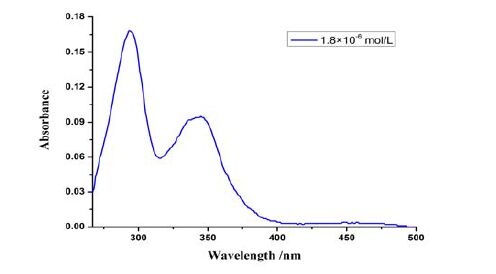
3.4 UV-Vis spectrum
UV-vis absorption spectra of compound II in the DMF solution with the concentration of 1.8 × 10-6 mol/L are investigated in Fig. 5. The UV-Vis spectrum of compound II in the DMF solution shows that compound II has two obvious absorption peaks at 294 and 345 nm. They are mainly caused by π···π* transition and n···π* transition, of which 294 nm for π···π* transition and 345 nm for n···π* transition. λex = 294 nm is the characteristic absorption peak of substitution in the quinolone of the parent nucleus.
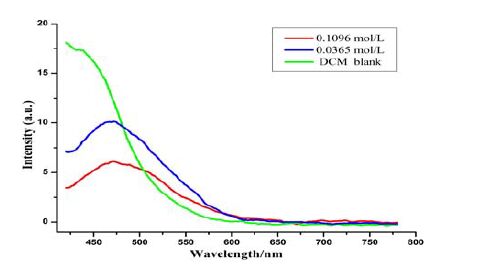
3.5 Photoluminescent properties and absolute quantum yield
The luminescent property of compound II was investigated. As illustrated in Fig. 6, weak fluorescence is found in the DCM solution. A blue fluorescent light peak at 470 nm can be found with excitation at 400 nm. Due to the self-quenching effect at higher concentration, fluorescence intensity was weaker at the high concentration of 0.1096 mol/L than that at 0.0365 mol/L.
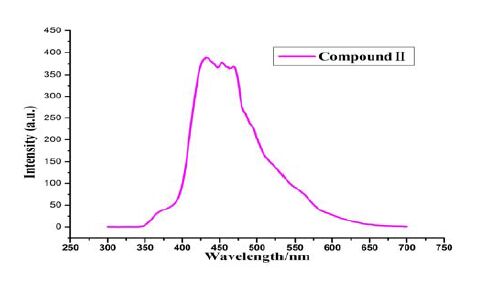
The solid-state luminescent property of compound II was further investigated at room temperature. As is shown in Fig. 7, a strong blue fluorescent light peak at 437 nm can be found with excitation at 202 nm.
Obviously, the fluorescence can be ascribed to the intramolecular π-π* transition of compound II. Compound II has a rigid planar structure with a large delocalized π bond, and has the lowest π-π* excitation energy. However, electron-withdrawing substituents -COOH, -C=O and halogen of compound II may also decrease the fluorescence intensity. We also found that fluorescence intensity in solid state is stronger than that in the DCM solution. The reason may be that the accumulation of molecules in the crystal mode also plays an enhanced role in their fluorescence in solid state. Furthermore, the maximum emission wavelength of solid stateis longer than that in the DCM solution; this phenomenon uncovers that solvent effect caused a blue shift. It is estimated that solid-state compound II has good molecular planarity, easier stacking, stronger intermolecular force and narrower band gap, thus causing a red shift. However, solvent effect made intermolecular force weaker and band gap wider, and then caused blue shift. In brief, these observations indicate that compound II may be an excellent candidate for potential photoactive materials.
Absolute fluorescence quantum yield of compound II hydrochloride in water solution with the concentration of 1.8×10-6 mol/L is 5.95% with the excitation at 409.9 nm.
3.6 Antibacterial activity
Table 3 obviously shows that the tested FQs, particularly CF·HCl and compound I, present strong antibacterial activities against most of the four strains. Delightfully, compound II has almost equal strong activity in comparison with CF·HCl and compound I, but stronger than NF nicotinic acid salt. Inspired by the activity of compound II, other modified compounds of compound II will be prepared soon, for obtaining more target molecules with excellent bioactivities.
Compound MIC (μg/mL) Inhibition zone diameter (mm)* S. aureus Salmonella P. aeruginosa E.coli S. aureus Salmonella P. aeruginosa E.coli CF·HCl 1 1 0.125 2 14 18 20 10 NF·Nicotinic >16 16 4 >16 -- 14 16 -- Compd.I 2 2 1 4 20 19 22 -- Compd II 8 8 2 >16 10 17 22 10 1) MIC: minimum inhibitory concentration; 2) * Drug concentration is 2 μg/mL, “--” indicates the diameter of inhibition zone less than 5 mm Table 3. Antimicrobial Activities (Expressed as MIC Values) in Vitro of Compound II4 CONCLUSION
In summary, a simple method for the preparation of compound II and its single crystal has been established. The crystallography belongs to the triclinic P1 space group. Compound II anhydrate forms a layered structure, and these layers are stacked with π···π interactions. The π-stacked layers are established along the crystallographic a-axis. XRPD, TG, UV-Vis spectrum, photoluminescent properties, absolute quantum yield and antibacterial activity of compound II were also studied preliminarily. Crystalline compound II demonstrates aggregation-induced fluorescence enhancement with an emission peak at 437 nm excited at 202 nm which may be due to the fact of intermolecular packing in the crystal structure. To our excitement, antibacterial activity uncovers that compound II presents excellent antibacterial activities against most of the four strains. This will stimulate us to prepare more target compounds for the exploration of structure-activity relationship.
-
-
[1]
Basavoju S., Bostrom D., Velaga S' P.. Pharmaceutical cocrystal and salts of norfloxacin[J]. Cryst' Growth Des', 2006, 6: 2699-2708. doi: 10.1021/cg060327x
-
[2]
Martínez-Alejo J' M., Dominguez-Chavez J' G., Rivera-Islas J., Herrera-Ruiz D., Höpfl H., Morales-Rojas H., Senosiain J' P.. A twist in cocrystals of salts: changes in packing and chloride coordination lead to opposite trends in the biopharmaceutical performance of eluoroquinolone hydrochloride cocrystals[J]. Cryst' Growth Des', 2014, 14: 3078-3095. doi: 10.1021/cg500345a
-
[3]
Sha J' Q., Li X., Zhou Y' H., Yan P' F., Li G' M., Wang C.. The introduction of antibacterial drug pipemidic acid into the POM field: syntheses, characterization and antitumor activity[J]. Solid State Sci', 2011, 13: 1972-1977. doi: 10.1016/j.solidstatesciences.2011.08.027
-
[4]
Bailey Walsh, R' D., Bradner M' W., Fleischman S., Morales L' A., Moulton B., Rodríguez-Hornedo N., Zaworotko M' J.. Crystal engineering of the composition of pharmaceutical phases[J]. Chem' Commun', 2003, 2: 186-187.
-
[5]
Karanam M., Choudhury A' R.. Structural landscape of pure enrofloxacin and its novel salts: enhanced solubility for better pharmaceutical applicability[J]. Cryst' Growth Des', 2013, 13: 1626-1637. doi: 10.1021/cg301831s
-
[6]
Reddy J' S., Ganesh S' V., Nagalapalli R., Dandela R., Solomon K' A., Kumar K' A., Goud N' R., Nangia A.. Fluoroquinolone salts with carboxylic acids[J]. J' Pharm' Sci', 2011, 100: 3160-3176. doi: 10.1002/jps.22537
-
[7]
Sha J' Q., Liang L' Y., Yan P' F., Li G' M., Wang C., Ma D' Y.. Study on ligation of copper complexes of the quinolone antibacterial drugs and octamolybdates POMs[J]. Polyhedron, 2012, 31: 422-430. doi: 10.1016/j.poly.2011.09.029
-
[8]
Drevenšek P., Zupancic T., Pihlar B., Jerala R., Kolitsch U., Plaper A., Turel I.. Mixed-valence Cu(II)/Cu(I) complex of quinolone ciprofloxacin isolated by a hydrothermal reaction in the presence of L-histidine: comparison of biological activities of variouscopper-ciprofloxacin compounds[J]. J' Inorg' Biochem', 2005, 99: 432-442. doi: 10.1016/j.jinorgbio.2004.10.018
-
[9]
Cheng G' Y., Hao H' H., Dai M' H., Liu Z' L., Yuan Z' H.. Antibacterial action of quinolones: from target to network[J]. Eur' J' Med' Chem', 2013, 66: 555-562. doi: 10.1016/j.ejmech.2013.01.057
-
[10]
Lins Gisele O' W., Campo L' F., Rodembusch F' S., Stefani V.. Novel ESIPT fluorescent benzazolyl-4-quinolones: synthesis, spectroscopic characterization and photophysical properties[J]. Dyes and Pigments, 2010, 84: 114-120. doi: 10.1016/j.dyepig.2009.07.010
-
[11]
Srivastava B' K., Solanki M., Mishra B., Soni R., Jayadev S., Valani D., Jain M., Patel P' R.. Synthesis and antibacterial activity of 4,5,6,7- tetrahydro-thieno[J]. Bioorg' Med' Chem' Lett', 2007, 17: 1924-1929. doi: 10.1016/j.bmcl.2007.01.038
-
[12]
Patel M' N., Bhatt B' S., Dosi P' A.. Topoisomerase inhibition, nucleolytic and electrolytic contribution on DNA binding activity exerted by biological active analogue of coordination compounds[J]. Appl' Biochem' Biotechnol', 2012, 166: 1949-1968. doi: 10.1007/s12010-012-9623-x
-
[13]
Andriole V' T.. The quinolones: past, present and future[J]. Clin' Infect' Dis', 2005, 41: 113-119. doi: 10.1086/428051
-
[14]
Mitton-Fry M' J., Brickner S' J., Hamel J' C., Brennan L., Casavant J' M., Chen M., Chen T., Ding X' Y., Driscoll J., Hardink J., Hoang T., Hua E., Huband M' D., Maloney M., Marfat A., McCurdy S' P., McLeod D., Plotkin M., Reilly U., Robinson S., Schafer J., Shepard R' M., Smith J' F., Stone G' G., Subramanyam C., Yoon K., Yuan W., Zaniewski R' P., Zook C.. Novel quinoline derivatives as inhibitors of bacterial DNA gyrase and topoisomerase IV' Bioorg[J]. Med' Chem' Lett', 2013, 23: 2955-2961. doi: 10.1016/j.bmcl.2013.03.047
-
[15]
Shou K' J., Li J., Jin Y., Lv Y' W.. Design, synthesis, biological evaluation, and molecular docking studies of quinolone derivatives as potential antitumor topoisomerase I inhibitors[J]. Chem' Pharm' Bull', 2013, 61: 631-636. doi: 10.1248/cpb.c13-00040
-
[16]
Williams G' M., Brunnemann K' D., Smart D' J., Molina D., Jeffrey A' M., Duan J' D., Krebsfaenger N., Kampkoetter A., Schmuck G.. Relationship of cellular topoisomerase II ainhibition to cytotoxicity and published genotoxicity of fluoroquinolone antibiotics in V79 cells[J]. Chem' Biol' Interact', 2013, 203: 386-390. doi: 10.1016/j.cbi.2013.01.003
-
[17]
Long Q' X., He Y., Xie J' P.. The molecular physiological and genetic mechanisms underlying the superb efficacy of quinolones[J]. Acta pharm' Sinica, 2012, 47: 969-977.
-
[18]
Gopalakrishnan A., Shanmugasundaram A., Yeon T' J.. Synthesis crystal and antibacterial studies of diversely functionalized tetrahydropyridin-4-ol[J]. Bioorg' Med' Chem' Lett', 2010, 20: 2242-2249. doi: 10.1016/j.bmcl.2010.02.015
-
[19]
He P' H., Guo D' H., Zhou X' Q.. Current status of fourth generation quinolone[J]. Chin' New Drugs J', 2004, 13: 1102-1105.
-
[20]
Lv R' Q., Xi Y' B., Lv J' L., Xu G' Y.. Advances in the fourth-generation fluorquinolone antibacterial agents[J]. Chin 'New Drugs J', 2012, 21: 1485-1493.
-
[21]
He J' H., Xiao D' R., Yan S' W., Sun D' Z., Chen H' Y., Wang X., Yang J., Ye Z' L., Yuan R., Wang E' B.. A series of novel 1D coordination polymers constructed from metal-quinolone complex fragments linked by aromatic dicarboxylate ligands[J]. Solid State Sci', 2012, 14: 1203-1210. doi: 10.1016/j.solidstatesciences.2012.06.004
-
[22]
He J' H., Sun D' Z., Xiao D' R., Yan S' W., Chen H' Y., Wang X., Yang J., Wang E' B.. Syntheses and structures of five 1D coordination polymers based on quinolone antibacterial agents and aromatic polycarboxylate ligands[J]. Polyhedron, 2012, 42: 24-29. doi: 10.1016/j.poly.2012.04.022
-
[23]
He J' H., Xiao D' R., Chen H' Y., Sun D' Z., Yan S' W., Wang X., Ye Z' L., Luo Q' L., Wang E' B.. A series of 2D metal-quinolone complexes: syntheses, structures, and physical properties[J]. J' Solid State Chem', 2013, 198: 279-288. doi: 10.1016/j.jssc.2012.10.015
-
[24]
Wang X., Yan S' W., Yang J., Xiao D' R., Zhang H' Y., Zhang J' L., Chi X' L., Wang E' B.. Three interdigitated metal-quinolone complexes from self-assembly of mixed ligands and cadmium salts[J]. Inorg' Chim' Acta, 2014, 409: 208-215. doi: 10.1016/j.ica.2013.09.031
-
[25]
Xu J' N., Tu S' J., Jiang H., Zhang J' P., Zhu X' T., Wang Q.. Synthesis and crystal structure of 3,7,7-trimethyl-1-phenyl- 4-(3-nitrophenyl)-4,5,6,7,8,9-hexahydro-1H-pyrazolo[J]. Chin' J' Struct' Chem', 2005, : 209-213.
-
[26]
Ge Y' H., Sun H., Wang M' L.. Crystal structure and fluorescence property of antofloxacin[J]. J' Southeast Univ', 2011, 27: 449-451.
-
[27]
Dolomanov O' V., Bourhis L' J., Gildea R' J., Howard J' A' K., Puschmann H.. OLEX2: a complete structure solution, refinement and analysis program[J]. Appl' Cryst', 2009, 42: 339-341. doi: 10.1107/S0021889808042726
-
[28]
Sheldrick G' M.. A short history of SHELX[J]. Acta Cryst, 2008, A64: 112-122.
-
[29]
Martı´n , S.; Beitia, J. I.; Ugalde, M.; Vitoria, P.; Corte´ s, R.Diethyl N,N’-O-phenylenedioxamate. Acta Cryst. 2002, E58, o913-o915.
-
[1]
-
Table 1. Bond Length s (Å) for Compound II
Bond Dist. Cl(1)–C(6) 1.735(2) Cl(2)–C(20) 1.777(4) Cl(3)–C(21) 1.775(10) Cl(4)–C(21) 1.756(10) F(1)–C(8) 1.348(3) O(1)–C(10) 1.207(4) O(2)–C(10) 1.325(4) O(3)–C(3) 1.261(3) O(4)–C(18) 1.227(4) N(1)–C(1) 1.348(3) N(1)–C(5) 1.406(3) N(1)–C(11) 1.469(3) N(2)–C(7) 1.389(3) N(2)–C(14) 1.462(3) N(2)–C(16) 1.467(3) N(3)–C(15) 1.465(4) N(3)–C(17) 1.466(4) N(3)–C(18) 1.346(4) C(1)–C(2) 1.364(4) C(2)–C(3) 1.423(4) C(2)–C(10) 1.487(4) C(3)–C(4) 1.456(4) C(4)–C(5) 1.414(3) C(4)–C(9) 1.400(4) C(5)–C(6) 1.411(3) C(6)–C(7) 1.406(4) C(7)–C(8) 1.402(3) C(8)–C(9) 1.351(4) C(11)–C(12) 1.492(4) C(11)–C(13) 1.478(4) C(12)–C(13) 1.484(5) C(14)–C(15) 1.508(4) C(16)–C(17) 1.513(4) C(18)–C(19) 1.515(4) C(19)–C(20) 1.496(5) C(21)A–Cl(4)A 1.817(15) C(21)A–Cl(3)A 1.751(14) Table 2. Bond Angles (Å) for Compound II
Angle (°) C(1)–N(1)–C(5) 119.4(2) C(1)–N(1)–C(11) 115.4(2) C(5)–N(1)–C(11) 125.0(2) C(7)–N(2)–C(14) 117.4(2) C(7)–N(2)–C(16) 122.4(2) C(14)–N(2)–C(16) 111.5(2) C(15)–N(3)–C(17) 112.9(2) C(18)–N(3)–C(15) 119.9(3) C(18)–N(3)–C(17) 127.2(3) N(1)–C(1)–C(2) 125.2(2) C(1)–C(2)–C(3) 119.2(2) C(1)–C(2)–C(10) 118.4(3) C(3)–C(2)–C(10) 122.4(2) O(3)–C(3)–C(2) 122.9(2) O(3)–C(3)–C(4) 121.0(2) C(2)–C(3)–C(4) 116.1(2) C(5)–C(4)–C(3) 121.7(2) C(9)–C(4)–C(3) 118.0(2) C(9)–C(4)–C(5) 120.3(2) N(1)–C(5)–C(4) 118.0(2) N(1)–C(5)–C(6) 124.6(2) C(6)–C(5)–C(4) 117.4(2) C(5)–C(6)–Cl(1) 122.41(19) C(7)–C(6)–Cl(1) 115.18(18) C(7)–C(6)–C(5) 122.2(2) N(2)–C(7)–C(6) 119.7(2) N(2)–C(7)–C(8) 123.7(2) C(8)–C(7)–C(6) 116.5(2) F(1)–C(8)–C(7) 118.4(2) F(1)–C(8)–C(9) 118.4(2) C(9)–C(8)–C(7) 123.1(2) C(8)–C(9)–C(4) 119.9(2) O(1)–C(10)–O(2) 121.8(3) O(1)–C(10)–C(2) 123.2(3) O(2)–C(10)–C(2) 115.0(3) N(1)–C(11)–C(12) 118.1(2) N(1)–C(11)–C(13) 119.3(2) C(13)–C(11)–C(12) 59.9(2) C(13)–C(12)–C(11) 59.6(2) C(11)–C(13)–C(12) 60.5(2) N(2)–C(14)–C(15) 109.3(2) N(3)–C(15)–C(14) 109.8(2) N(2)–C(16)–C(17) 108.8(2) N(3)–C(17)–C(16) 110.7(2) O(4)–C(18)–N(3) 121.7(3) O(4)–C(18)–C(19) 120.1(3) N(3)–C(18)–C(19) 118.1(3) C(20)–C(19)–C(18) 110.7(3) C(19)–C(20)–Cl(2) 111.3(3) Cl(4)–C(21)–Cl(3) 112.5(5) Cl(3)A–C(21)A–Cl(4)A 110.4(9) Table 3. Antimicrobial Activities (Expressed as MIC Values) in Vitro of Compound II
Compound MIC (μg/mL) Inhibition zone diameter (mm)* S. aureus Salmonella P. aeruginosa E.coli S. aureus Salmonella P. aeruginosa E.coli CF·HCl 1 1 0.125 2 14 18 20 10 NF·Nicotinic >16 16 4 >16 -- 14 16 -- Compd.I 2 2 1 4 20 19 22 -- Compd II 8 8 2 >16 10 17 22 10 1) MIC: minimum inhibitory concentration; 2) * Drug concentration is 2 μg/mL, “--” indicates the diameter of inhibition zone less than 5 mm -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 3
- 文章访问数: 2965
- HTML全文浏览量: 167


 下载:
下载:







 下载:
下载:

