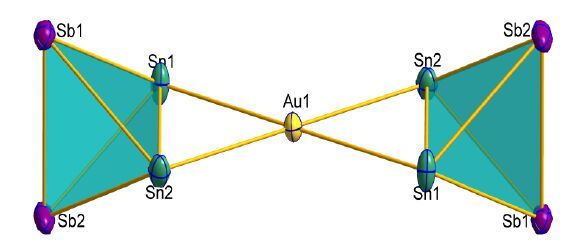
 图1
Thermal ellipsoid plot of the molecular cluster anion 1a (drawn at 50% probability)
Figure1.
Thermal ellipsoid plot of the molecular cluster anion 1a (drawn at 50% probability)
图1
Thermal ellipsoid plot of the molecular cluster anion 1a (drawn at 50% probability)
Figure1.
Thermal ellipsoid plot of the molecular cluster anion 1a (drawn at 50% probability)

[Au(η2-Sn2Sb2)2]3-: a Mixed Group 14/15 Tetrahedral Dimer Bridged by Au①
English
[Au(η2-Sn2Sb2)2]3-: a Mixed Group 14/15 Tetrahedral Dimer Bridged by Au①
-
Key words:
- zintl cluster
- / DFT calculations
- / gold
- / antimony
-
1 INTRODUCTION
During the past decades, transition or rare-earth metal comprised Zintl clusters with unique geometries and diverse electronic structures have been obtained through solution chemistry synthesis and gas phase experiments, some of which have been studied in the fields of catalysis and materials science[1, 2]. Deltahedrals of group 14/15 elements, bridged by transition metals, play an important role in Zintl clusters[3]. In the case of E9 (E = Ge, Sn, Pb) units, transition metal connected dimers, oligomers and one-dimensional strands are common structures. Selected examples include dimers [(Ni-Ni- Ni)@(Ge9)2]4-, [Au3(Ge9)2]5-, oligomer[Hg3(Ge9)4]10- and one-dimensional strand 1∞[HgGe9]2-[4-7]. As for group 15 elements, the [Pn7]3- units[2a] usually form dimers linked by transition metals[2b], such as [Zn (Pn7)2]4- (Pn = P, As) and [M2(As7)2]4- (M = Cu, Hg, Pd, Au)[8-12]. Some other metal bridged models like [Bi-Zn-Bi]4-, [Cd2Sb5]11-, 1∞[CdSb2]4- and Ba13Si6Sn8As22 were also reported[13-17].
In contrast with the aforementioned studies, tetrahedral (E4) units coordinated by transition metals are usually isolated from high temperature solid-state reactions instead of solution techniques, such as [Zn (Ge4)2]6-, [Cd3(Pb4)4]10-, 1∞[{Au (E4)}4-] (E = Ge, Sn, Pb) and i ts ana logue 1∞[{Au (TlSn3)}4-][18-23]. The reaction chemistry of binary Zintl compound A4E4 (A = Na, K, Rb, Cs) has been limited because of the insolubility in common solvents like N, N-dimethylformamide (dmf) and ethylenediamine (en)[19, 24, 25]. With the extensive ion pairing, liquid ammonia is so far the only solvent to stabilize highly charged [E4]4- and their derivatives [(MesCu)2(η3-E4)]4- (E = Si, Ge, Si/Ge), [Zn{η2-(Si/Ge)4}2]6-, [(η2-Sn4) Zn (η3-Sn4)]6- and [Au (η2-Sn4)2]7-[19, 24-30].
Until now, several mixed group 14/15 tetrahedral (E2/Pn2) anions have been reported, such as [Sn2Sb2]2-, [Sn2Bi2]2- and [Pb2Bi2]2-[31-33], however, the dimers of these units still remain unexplored. We have focused our interest on the synthesis of Zintl clusters, such as the all-metal sandwich cluster [Sb3Au3Sb3]3-[34]. In this paper, we report the first Au-Sn-Sb Zintl cluster [Au (η2-Sn2Sb2)2]3- (1a; Fig. 1) in the form of [K (2, 2, 2-crypt)]3[Au (η2-Sn2Sb2)2] (1), in which two [Sn2Sb2]2- units are bridged by one gold atom.
 图1
Thermal ellipsoid plot of the molecular cluster anion 1a (drawn at 50% probability)
Figure1.
Thermal ellipsoid plot of the molecular cluster anion 1a (drawn at 50% probability)
图1
Thermal ellipsoid plot of the molecular cluster anion 1a (drawn at 50% probability)
Figure1.
Thermal ellipsoid plot of the molecular cluster anion 1a (drawn at 50% probability)
2 EXPERIMENTAL
All manipulations and reactions were performed in a nitrogen atmosphere using standard Schlenk or glovebox techniques. All solvents were freshly distilled and stored under nitrogen prior to use. 2, 2, 2-crypt (TCI, 98%) was dried in vacuum for one day. [K (2.2.2-crypt)]2[Sn2Sb2]·en and (PPh3) AuPh were prepared according to literature[31, 35, 36]. Deuterated solvent C6D6 (Alfa Aesar) was dried over activated molecular sieves (4 Å) and vacuum transferred before use, while dmf-d7 (Alfa Aesar) was directly used without further purification. EDX analysis on 1 (Fig. S2 and Table S2) was performed on the Energy Disperse Spectroscopy (EDX). Spectra were obtained by using a scanning electron microscope (Hitachi S-4800) equipped with a Bruker AXS XFlash detector 4010. The 119Sn NMR spectra were recorded on a Bruker DRX500 AVANCE spectrometer at 186.5 MHz at 298 K in dmf-d7. Before dissolving in dmf-d7 solvent, the crystals of 1 were washed with toluene and dried in vacuum.
2.1 Synthesis of 1
In a 10 mL vial, 137 mg (0.10 mmol) of [K (2.2.2- crypt)]2[Sn2Sb2]·en was dissolved in ethylenediamine (2 ml). In a second vial, 40 mg (0.07 mmol) (PPh3) AuPh was dissolved in 0.5 ml toluene. The toluene solution was added to ethylenediamine solution dropwise while stirring powerfully. The mixture was allowed to stir for 4 h at room temperature. The resulting dark red solution was subsequently filtered through glass wool and transferred to a test tube, then carefully layered by toluene (2.5 mL). After 3 days, dark red crystals of 1 with moderate yield (46% based on Au precursor) appeared on the interfaces of the tube wall.
2.2 Structure determination
Single-crystal X-ray diffraction data of 1 were collected on a Bruker Apex II CCD diffractometer with graphite-monochromated MoKα radiation (λ = 0.71073 Å) at 123 K. Data processing was accomplished with the SAINT program[37]. The structure was solved by direct methods with SHELXS-97 program[38a] and refined on F2 by full-matrix leastsquares method with anisotropic thermal parameters for all non-hydrogen atoms using SHELXL-97[38b]. The non-hydrogen atoms were refined anisotropically, and hydrogen atoms were added according to theoretical models. Out of the 21107 total reflections collected in the range of 1.56≤θ≤ 25.03º by using an ω-φ scan mode, 7632 were independent with Rint = 0.0368, of which 6370 were observed with I > 2σ(I) and used in the structure determination and refinements. The final full-matrix least-squares refinement gave R = 0.0571, wR = 0.1466 (w = 1/[σ2(Fo 2) + (0.0717P)2 + 173.3675P], where P = (Fo 2 + 2Fc 2)/3), S = 1.046, (Δ/σ) max = 0.001, (Δρ) max = 3.482 and (Δρ) min = -2.087 e/Å3.
3 RESULTS AND DISCUSSION
Complex 1 was directly extracted from the reaction of [K (2.2.2-crypt)]2[Sn2Sb2]·en and (PPh3) AuPh in ethylenediamine at room temperature. The molecular structure of 1 was characterized by single-crystal X-ray diffraction. The monoclinic crystal system with C2/c space group has the crystallographic data as follows: a = 14.2109(8), b = 14.2109(8), c = 27.3036(15) Å, β = 106.7290(10)° and V = 8659.5(8) Å3. The elemental ratio of K, Au, Sn and Sb has been confirmed by energy-dispersive X-ray spectroscopy analysis (the ratio of K/Au/Sn/Sb is approximately 3:1:4:4, Supporting Information, Fig. S2 and Table S2).
In this dimer, one Au+ is coordinated by two [Sn2Sb2]2- tetrahedral edges. The mean bond length of Au-Sn in 1a is 2.75 Å, which is in accordance with those in [Au (η2-Sn4)2]7- (2.78 Å) and NaAuSn (2.75 Å)[30, 39]. The Sn-Sn distance is 3.12 Å, which is elongated compared with normal Sn-Sn single bond (2.80 Å in α-Sn), but similar with those in [Au (η2-Sn4)2]7- (3.06 Å) and [Pd2@Sn18]4- (3.01~ 3.15 Å)[30, 40, 41]. The Sn-Sb distances range from 2.86 to 2.88 Å and the Sb-Sb distance is 2.81 Å, which are akin to those in [Sn2Sb5(ZnPh)2]3- (2.84~2.96 and 2.79~2.82 Å, respectively)[31]. In addition, the Sb-Sb distance is slightly shorter than those in [Ni5Sb17]4- (2.82 ~ 2.93 Å) and [(CO)3Ni3Sb7]3- (2.84~2.98), but longer than the mean distances in [NbSb8]3- (2.78 Å) and the transition metal-free [Sb4]2- (2.75 Å)[42-45]. Contrary to the precursor [Sn2Sb2]2- (2.85~2.88 Å) which is almost a regular tetrahedron, [Sn2Sb2]2- in 1a is obviously distorted. As shown in Table 1, three tetrahedral coordination fashions η3-M-η3, η3-M-η2, η2-M-η2 have been reported, and 1a belongs to the third type. These coordination fashions represent three different structures, which can be recognized from the front and side views. Among the η2-M-η2 manner cluster anions [Au (η2-Sn4)2]7-[30], [Zn{η2- (Si/Ge)4}2]6-[25] and 1a, a significant structural difference of 1a is that the two AuSn2 faces are coplanar, while the corresponding AuSn2 and Zn (Si/Ge)2 planes in [Au (η2-Sn4)2]7- and [Zn{η2- (Si/Ge)4}2]6- are close to vertical. Another important difference is reflected in the coordination environment of the dimers. [Au (η2-Sn4)2]7- and Zn{η2- (Si/Ge)4}2]6- are further linked by K+ ammoniate, while 1a is a discrete cluster anion.
 Table 1.
Front and Side Views of Selected Cluster Anions: a) [Zn (η3-Ge4)2]6-; b) [(η3-E4) Zn (η2-E4)]6- (E = Ge, Sn); c) [Zn{η2-(Si/Ge)4}2]6-, [Au (η2-Sn4)2]7-; d) 1a
Table 1.
Front and Side Views of Selected Cluster Anions: a) [Zn (η3-Ge4)2]6-; b) [(η3-E4) Zn (η2-E4)]6- (E = Ge, Sn); c) [Zn{η2-(Si/Ge)4}2]6-, [Au (η2-Sn4)2]7-; d) 1a
Formulas Front view Side view Ref. a) [M(η3-Ge4)2]6- 

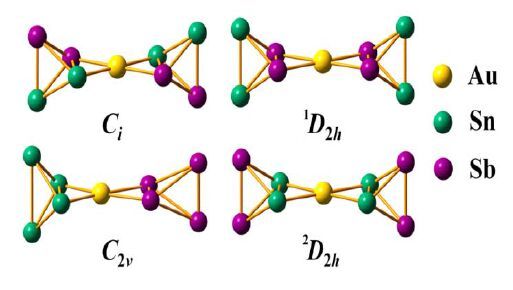
18 b) [(η3-E4)M(η2-E4)]6- 19, 25 c) [M(η2-E4)2]n- 24, 30 d) 1a This work However, question arises. Why Au (I) is only coordinated by Sn atoms instead of co-occupied Sn/Sb atoms with statistical occupation ratios? Previous works have shown that some group 14/15 atoms in heteroatomic Zintl ions like [Sn2Bi2]2-[32], [Pb2Bi2]2-[33], [Sn2Sb2]2-[31] and most of the m-14-15 clusters such as M/Ln-Sn-Bi (M/Ln = Zn, Ni, La, Ce, Eu) are statistic disorder, and their positions could not be confirmed by single-crystal X-ray diffraction[46-49], except the "fully ordered" [Sn2Bi2]2- and [Sn4Bi4]4- which are obtained from liquid ammonia[50]. Theoretical calculations provide insight into the distribution situation studies of group 14/15 atoms over each position. In this work, quantum chemical calculations employing density functional theory methods (DFT) were performed to explore the stability and electronic structure of 1a. An exhaustive survey of all possible isomers of 1a has been conducted to identify the certain positions of Sn/Sb atoms (Fig. 2).
In order to confirm the structure of 1a, we optimized the isomers by different functions, such as B3LYP, M06, wB97X-D, and SCS-MP2 together with DeF2-TZVP calculated in the Gaussian09 program package (Table S3)[51-57]. Herein, we list the electronic energy and the energy gap values in Table 2 calculated by B3LYP function. Previous work has shown good performance of B3LYP on the studies of this kind of clusters[19, 24]. As shown in Table 2, the electronic energy of the isomer in which Au is coordinated by four Sn atoms is a stable minimum, demonstrating that the 2D2h conformation is the best candidate for the ground state. Furthermore, the calculated energy gap and electronic energy values also follow the same trends. The energy gap value of this isomer is 2.76 eV, which is the largest among isomers. Accordingly, 2D2h conformation is the exclusive existent stable structure.
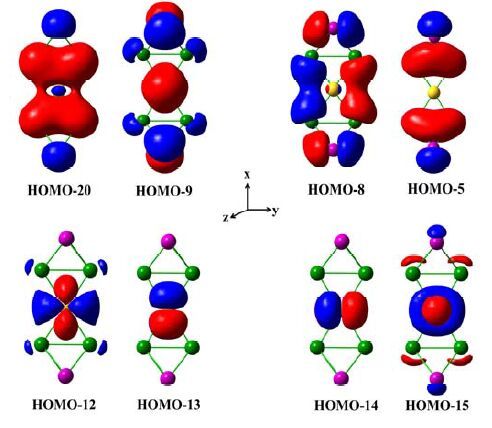
Symmetry Relative energy/kJ mol-1 Energy gap/eV Ci 48 2.51 1D2h 92 2.22 C2v 291 1.25 2D2h 0 2.76 One Au+ binds two tetrahedral [Sn2Sb2]2- in η2 fashion so that the local coordination sphere of the Au atom is nearly planar. Because gold atom has a little more electronegativity than the tin atom, the charge distribution in [Au (η2-Sn2Sb2)2]3- with threefold negative charge is seriously concerned. We performed Natural Population Analysis (NPA) with Version 6.0 of the NBO program[58]. The gold atom shows slightly positive charge (+0.12), while the tin atoms in 1a carry -0.23 partial charge. In contrast, the antimony atoms in 1a carry more partial charges of -0.55, which is nearly consistent with the charge carried by isolated [Sn2Sb2]2- (-0.56). The result hints that charge-transfer takes place. Moreover, the observed planar conformation of the gold atom in 1a suggested d-orbital contributions to bonding. Indeed, the charge decomposition analysis (CDA)[59] showed the planar conformation is induced by electron donation and back-donation between Au atom and two coordinated tetrahedral [Sn2Sb2]2-. Remarkably, 0.3 e is donated from the coordinated tetrahedral [Sn2Sb2]2- to Au+, while 0.14 e is back-donated from Au+ to [Sn2Sb2]2-. According to CDA, HOMO-20, HOMO-9, HOMO- 8 and HOMO-5 contribute partially to electron donation from [Sn2Sb2]2- to Au+, whereas HOMO- 12, HOMO-13, HOMO-14 and HOMO-15 represent the major back donation from Au+ to [Sn2Sb2]2-. For HOMO-20, it is built up of 54% s by tin atoms and 28% by stibium atoms. The trend of charge transfer from gold atom to tin atoms can be easily viewed in Fig. 3. 68% of HOMO-9 is located on the stibium atoms by p orbital and 20% on tin atoms by p orbital. 42% of HOMO-8 is located on the tin atoms by s orbital and 54% on stibium atoms by p orbital. HOMO-12, HOMO-13, HOMO-14 and HOMO-15 show 86% dz 2, 98% dyz, 95% dxz and 91% dx 2 -y 2 orbital character by gold atom, respectively. In a word, electron donation and back donation are the key factors forming planar conformation of Au atom in 1a by orbital composition analysis, as outlined in the prior research[60]. Meanwhile, the calculated Mayer bond order of Au-Sn is 0.43, which is much smaller than those of Sb-Sb (1.04) and Sn-Sn (0.89), indicating relatively weaker interactions between Au and the coordinated tetrahedra, as noted by the earlier research[60].
4 CONCLUSION
In the synthesis of tetrahedral dimer, we have successfully extended the scope of solvents from liquid ammonia to ethylenediamine. In contrast to the previously reported Zintl clusters with tetrahedral dimer, 1a features a new structure type with coplanar AuSn2 faces. Quantum chemical calculations combined with 119Sn NMR spectra confirmed the Sn/Sb positions. The unusual D2h symmetric [Au (η2-Sn2Sb2)2]3- and the planar bonding situation are induced by electron donation and backdonation.
-
-
[1]
Cui L. F., Huang X., Wang L. M., Zubarev D. Y., Boldyrev A. I., Li J., Wang L. S.. Sn122-: stannaspherene[J]. J. Am. Chem. Soc., 2006, 128: 8390-8391. doi: 10.1021/ja062052f
-
[2]
(a) Dai, F. R.; Xu, L. Syntheses, crystal structures and properties of [K(2,2,2-crypt)]3K(HP7)2·en, [K(18-crown-6)]2HP7 and [K(db 18-crown-6)]2HP7·toluene. Inorg. Chim. Acta 2006, 359, 4265-4273. (b) Turbervill, R. S. P.; Goicoechea, J. M. From clusters to unorthodox pnictogen sources: solution-phase reactivity of [E7]3- (E = P-Sb) anions. Chem. Rev. 2014, 114, 10807-10828.
-
[3]
Scharfe , S , Kraus , F , Stegmaier , S , Schier , A , Fässler , T. F., Zintl ions. cage compounds, and intermetalloid clusters of group 14 and group 15 elements[J]. Angew. Chem., Int. Ed., 2011, 50: 3630-3671. doi: 10.1002/anie.v50.16
-
[4]
Goicoechea , J. M., Sevov , S. C.. [(Ni-Ni-Ni)@(Ge9)2]4-: a linear triatomic nickel filament enclosed in a dimer of nine-atom germanium clusters[J]. Angew. Chem., Int. Ed., 2005, 44: 4026-4028. doi: 10.1002/(ISSN)1521-3773
-
[5]
Spiekermann , A , Hoffmann , S. D., Kraus , F , Fässler , T. F.. [Au3Ge18]5-—A gold-germanium cluster with remarkable Au-Au interactions[J]. Angew. Chem., Int. Ed., 2007, 46: 1638-1640. doi: 10.1002/(ISSN)1521-3773
-
[6]
Denning , M. S., Goicoechea , J. M.. [Hg3(Ge9)4]10-: a nanometric molecular rod precursor to polymeric mercury-linked cluster chains[J]. Dalton Trans., 2008, : 5882-5885.
-
[7]
Nienhaus , A , Hauptmann , R , Fässler , T. F.. 1 ∞[HgGe9]2--a polymer with Zintl ions as building blocks covalently linked by heteroatoms[J]. Angew. Chem., Int. Ed., 2002, 41: 3213-3215. doi: 10.1002/1521-3773(20020902)41:17<>1.0.CO;2-C
-
[8]
Knapp C., Zhou B., Denning M. S., Rees N. H., Goicoechea J. M.. Reactivity studies of group 15 Zintl ions towards homoleptic post-transition metal organometallics: a ‘bottom-up’ approach to bimetallic molecular clusters[J]. Dalton Trans., 2010, 39: 426-436. doi: 10.1039/B911544G
-
[9]
Mandal S., Reber A. C., Qian M. C., Liu R., Saavedra H. M., Sen S., Weiss P. S., Khanna S. N., Sen A.. On the stability of an unsupported mercury-mercury bond linking group 15 Zintl clusters[J]. Dalton Trans., 2012, 41: 5454-5457. doi: 10.1039/c2dt30083d
-
[10]
Mandal S., Reber A. C., Qian M., Liu R., Saavedra H. M., Sen S., Weiss P. S., Khanna S. N., Sen A.. Synthesis, structure and band gap energy of covalently linked cluster-assembled materials[J]. Dalton Trans., 2012, 41: 12365-12377. doi: 10.1039/c2dt31286g
-
[11]
Moses M. J., Fettinger J. C., Eichhorn B. W.. Charged molecular alloys: synthesis and characterization of the binary anions Pd7As16 4- and Pd2As14 4-. J. Am[J]. Chem. Soc., 2002, 124: 5944-5945. doi: 10.1021/ja025627r
-
[12]
Chaki N. K., Mandal S., Reber A. C., Qian M., Saavedra H. M., Weiss P. S., Khanna S. N., Sen A.. Controlling band gap energies in cluster-assembled ionic solids through internal electric fields[J]. ACS Nano, 2010, 4: 5813-5818. doi: 10.1021/nn101640r
-
[13]
Qin Q., Zhou L., Wang Y., Sang R., Xu L.. Linear triatomic [ZnBi2]4- in K4ZnBi2.[J]. Dalton Trans., 2014, 43: 5990-5993. doi: 10.1039/c3dt53419g
-
[14]
Benda C. B., Köchner T., Schäper R., Schulz S., Fässler T. F.. Bi-Zn bond formation in liquid ammonia solution: [Bi-Zn-Bi]4-, a linear polyanion that is iso(valence)-electronic to CO2.[J]. Angew. Chem., Int. Ed., 2014, 53: 8944-8948. doi: 10.1002/anie.201404343
-
[15]
Wang Y., Qin Q., Sang R., Xu L.. K11Cd2Sb5 built of unprecedented planar CdSb3 triangle[J]. Dalton Trans., 2015, 44: 18316-18319. doi: 10.1039/C5DT02600H
-
[16]
Xia S. Q., Bobev S.. Cation anion interactions as structure directing factors: structure and bonding of Ca2CdSb2 and Yb2CdSb2. J. Am[J]. Chem. Soc., 2007, 129: 4049-4057. doi: 10.1021/ja069261k
-
[17]
Liu X. C., Lin N., Wang J., Pan M. Y., Zhao X., Tao X. T., Xia S. Q.. Ba13Si6Sn8As22: a quaternary Zintl phase containing adamantane-like [Si4As10] clusters[J]. Inorg. Chem., 2013, 52: 11836-11842. doi: 10.1021/ic402023z
-
[18]
Queneau V., Sevov S. C.. Cs6Ge8Zn: a zintl phase with isolated heteroatomic clusters of Ge8Zn[J]. J. Am. Chem. Soc., 1997, 119: 8109-8110. doi: 10.1021/ja9717121
-
[19]
Stegmaier S., Waibel M., Henze A., Jantke L. A., Karttunen A. J., Fässler T. F.. Soluble Zintl phases A14ZnGe16 (A = K, Rb) featuring [(η3-Ge4)Zn(η2-Ge4)]6- and [Ge4]4- clusters and the isolation of [(MesCu)2(η3,η3-Ge4)]4-: the missing link in the solution chemistry of tetrahedral group 14 element Zintl clusters[J]. J. Am. Chem. Soc., 2012, 134: 14450-14460. doi: 10.1021/ja304251t
-
[20]
Todorov E., Sevov S. C.. K6Pb8Cd: a zintl phase with oligomers of Pb4 tetrahedra interconnected by Cd atoms[J]. Angew. Chem., Int. Ed., 1999, 38: 1775-1777. doi: 10.1002/(ISSN)1521-3773
-
[21]
Zachwieja U., Wlodarski J.. Zintl-verbindungen mit gold und germanium: M3AuGe4 mit M = K, Rb und Cs[J]. Z. Anorg. Allg. Chem., 2004, 630: 993-997. doi: 10.1002/(ISSN)1521-3749
-
[22]
Zachwieja U., Müller J., Wlodarski J.. Zintl-verbindungen mit gold: M3AuSn4 mit M = K, Rb, Cs und M3AuPb4 mit M = Rb, Cs[J]. Z. Anorg. Allg. Chem., 1998, 624: 853-858. doi: 10.1002/(ISSN)1521-3749
-
[23]
Huang D., Corbett J. D.. K4Au(TlSn3): a novel zintl phase with an anionic chain[J]. Inorg. Chem., 1998, 37: 5007-5010. doi: 10.1021/ic980579q
-
[24]
Waibel , M , Henneberger , T , Jantke , L. A., Fässler , T. F.. [(η2-(Si/Ge)4)Zn(η2-(Si/Ge)4)]6-- novel zintl clusters with mixed Si/Ge tetrahedra bridged by a Zn atom[J]. Chem. Commun., 2012, 48: 8676-8678. doi: 10.1039/c2cc33652a
-
[25]
Fendt F., Koch C., Gärtner S., Korber N.. Reaction of Sn4 4- in liquid ammonia: the formation of Rb6[(η2-Sn4)Zn(η3-Sn4)]·5NH3.[J]. Dalton Trans., 2013, 42: 15548-15550. doi: 10.1039/c3dt51932e
-
[26]
Wiesler K., Brandl K., Fleischmann A., Korber N.. Tetrahedral [Tt4]4- Zintl anions through solution chemistry: syntheses and crystal structures of the ammoniates Rb4Sn4·2NH3, Cs4Sn4·2NH3, and Rb4Pb4·2NH3. Z. Anorg[J]. Allg. Chem., 2009, 635: 508-512. doi: 10.1002/zaac.v635:3
-
[27]
Neumeier M., Fendt F., Gärtner S., Koch C., Gärtner T., Korber N., Gschwind R. M.. Detection of the elusive highly charged zintl ions Si4 4- and Sn4 4- in liquid ammonia by NMR spectroscopy[J]. Angew. Chem., Int. Ed., 2013, 52: 4483-4486. doi: 10.1002/anie.201209578
-
[28]
Waibel , M , Kraus , F , Scharfe , S , Wa hl, B , Fässler , T. F.. [(MesCu)2(η3-Si4)]4-: a mesitylcopper-stabilized tetrasilicide tetraanion[J]. Angew. Chem., Int. Ed., 2010, 49: 6611-6615. doi: 10.1002/anie.201002153
-
[29]
Waibel M., Sieber G. R., Fässler T. F.. Heteroatomic Si/Ge zintl clusters: single-crystal structure determination of Rb4[Si7.8Ge1.2](NH3)5 and [Rb([18]crown-6)Rb3][Si7.5Ge1.5](NH3)4.[J]. Chem. -Eur. J., 2011, 17: 13391-12931. doi: 10.1002/chem.v17.48
-
[30]
Benda C. B., Waibel M., Köchner T., Fässler T. F.. Reactivity of liquid ammonia solutions of the zintl phase K12Sn17 towards mesitylcopper(I) and phosphinegold(I) chloride[J]. Chem. -Eur. J., 2014, 20: 16738-16746. doi: 10.1002/chem.201404594
-
[31]
Lips F., Schellenberg I., Pöttgen R., Dehnen S.. The subtle influence of binary versus homoatomic zintl ions: the phenyl-ligated trimetallic cage [Sn2Sb5(ZnPh)2]3-.[J]. Chem. -Eur. J., 2009, 15: 12968-12973. doi: 10.1002/chem.v15:47
-
[32]
Critchlow S. C., Corbett J. D.. Heteropolyatomic anions of the post transition metals[J]. Synthesis and structure of the ditindibismuthide(2 -) anion, Sn2Bi2 2-. Inorg. Chem., 1982, 21: 3286-3290.
-
[33]
Ababei R., Heine J., Ho?ynska M., Thiele G., Weinert B., Xie X., Weigend F., Dehnen S.. Making practical use of the pseudo-element concept: an efficient way to ternary intermetalloid clusters by an isoelectronic Pb--Bi combination[J]. Chem. Commun., 2012, 48: 11295-11297. doi: 10.1039/c2cc35318k
-
[34]
Pan F. X., Li L. J., Wang Y. J., Guo J. C., Zhai H. J., Xu L., Sun Z. M.. An all-metal aromatic sandwich complex [Sb3Au3Sb3]3-. J. Am[J]. Chem. Soc., 2015, 137: 10954-10957. doi: 10.1021/jacs.5b07730
-
[35]
Coates G. E., Parkin C.. Gold(I) alkynyls and their coordination complexes[J]. J. Chem. Soc., 1962, : 3220-3226. doi: 10.1039/jr9620003220
-
[36]
Hong X., Cheung K. K., Guo C. X., Che C. M.. Luminescent organometallic gold(I) complexes[J]. Structure and photophysical properties of alkyl-, aryl- and μ-ethynylene gold(I) complexes. J. Chem. Soc. Dalton. Trans., 1994, : 1867-1871.
-
[37]
SMART and SAINT (software packages). Siemens Analytical X-ray Instruments, Inc., Madison, WI 1996.
-
[38]
(a) Sheldrick, G. M. SHELXS-97, Program for the Solution of Crystal Structure. University of Göttingen, Germany 1997. (b) Sheldrick, G. M. SHELXL-97, Program for Crystal Structure Solution and refinement. University of Göttingen, Germany 1997.
-
[39]
Wrobel G., Schuster H. U.. Die kristallstrukturen der phasen Na2AuGe und NaAuSn[J]. Z. Anorg. Allg. Chem., 1977, 432: 95-100. doi: 10.1002/(ISSN)1521-3749
-
[40]
Sun Z. M., Xiao H., Li J., Wang L. S.. Pd2@Sn18 4-: fusion of two endohedral stannaspherenes[J]. J. Am. Chem. Soc., 2007, 129: 9560-9561. doi: 10.1021/ja0728674
-
[41]
Kocak F. S., Zavalij P., Lam Y. F., Eichhorn B. W.. Solution dynamics and gas-phase chemistry of Pd2@Sn18 4-. Inorg[J]. Chem., 2008, 47: 3515-3520.
-
[42]
Moses , M. J., Fettinger , J. C., Eichhorn , B. W.. [Ni5Sb17]4- transition-metal zintl ion complex: crossing the Zintl border in molecular intermetalloid clusters[J]. Inorg. Chem., 2007, 46: 1036-1038. doi: 10.1021/ic0616535
-
[43]
Charles S., Eichhorn B. W., Bott S. G.. Synthesis and structure of [Sb7Ni3(CO)3]3-: a new structural type for nido 10-vertex polyhedral clusters[J]. J. Am. Chem. Soc., 1993, 115: 5837-5838. doi: 10.1021/ja00066a067
-
[44]
Kesanli B., Fettinger J., Scott B., Eichhorn B. W.. Gas phase, solution, and solid state alkali ion binding by the [NbE8]3- (E = As, Sb) complexes: synthesis, structure, and spectroscopy[J]. Inorg. Chem., 2004, 43: 3840-3846. doi: 10.1021/ic035397x
-
[45]
Critchlow S. C., Corbett J. D.. Homopolyatomic anions of the post transition elements[J]. Synthesis and structure of potassium-crypt salts of the tetraantimonide(2-) and heptaantimonide(3-) anions, Sb4 2- and Sb7 3-. Inorg. Chem., 1984, 23: 770-774.
-
[46]
Li, F, Dehnen, S. [Zn6Sn3Bi8]4-: expanding the intermetalloid zintl anion concept to ternary systems. Angew[J]. Chem., Int. Ed., 2009, 48: 6435-6438. doi: 10.1002/anie.v48:35
-
[47]
Lips F., Dehnen S.. Neither electron-precise nor in accordance with Wade-Mingos rules: the ternary cluster anion [Ni2Sn7Bi5]3-. Angew[J]. Chem., Int. Ed., 2011, 50: 955-959. doi: 10.1002/anie.201005496
-
[48]
Lips F., Ho?yńska M., Clérac R., Linne U., Schellenberg I., Pottgen R., Weigend F., Dehnen S.. Doped semimetal clusters: ternary, intermetalloid anions [Ln@Sn7Bi7]4- and [Ln@Sn4Bi9]4- (Ln = La, Ce) with adjustable magnetic properties[J]. J. Am. Chem. Soc., 2012, 134: 1181-1191. doi: 10.1021/ja209226b
-
[49]
Li, F, Clérac, R, Dehnen, S. [Eu@Sn6Bi8]4-: a mini-fullerane-type Zintl anion containing a lanthanide ion[J]. Angew. Chem., Int. Ed., 2011, 50: 960-964. doi: 10.1002/anie.201005655
-
[50]
Friedrich U., Neumeier M., Koch C., Korber N.. Synthesis of heteroatomic Zintl anions in liquid ammonia - the new highly charged [Sn4Bi4]4- and fully ordered [Sn2Bi2]2-.[J]. Chem. Commun., 2012, 48: 10544-10546. doi: 10.1039/c2cc35380f
-
[51]
Stephens P. J., Devlin F. J., Chabalowski C. F., Frisch M. J.. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields[J]. J. Phys. Chem., 1994, 98: 11623-11627. doi: 10.1021/j100096a001
-
[52]
Zhao Y., Truhlar D. G.. The M 06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M 06-class functionals and 12 other functionals[J]. Theor. Chem. Acc., 2008, 120: 215-241. doi: 10.1007/s00214-007-0310-x
-
[53]
Chai J. D., Head-Gordon M.. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections[J]. Phys. Chem. Chem. Phys., 2008, 10: 6615-6620. doi: 10.1039/b810189b
-
[54]
Frisch M. J., Head-Gordon M., Pople J. A.. Semi-direct algorithms for the MP 2 energy and gradient[J]. Chem. Phys. Lett., 1990, 166: 281-289. doi: 10.1016/0009-2614(90)80030-H
-
[55]
Frisch M. J., Head-Gordon M., Pople J. A.. A direct MP 2 gradient method[J]. Chem. Phys. Lett., 1990, 166: 275-280. doi: 10.1016/0009-2614(90)80029-D
-
[56]
Weigend F., Ahlrichs R.. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy[J]. Phys. Chem. Chem. Phys., 2005, 7: 3297-3305. doi: 10.1039/b508541a
-
[57]
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery Jr., J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M. J.; Knox, E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski J.; Fox, D. J. GAUSSIAN 09 (Revision B.01), Gaussian, Inc., Wallingford, CT 2010.
-
[58]
Glendening, E. D.; Badenhoop, J. K.; Reed, A. E.; Carpenter, J. E.; Bohmann, J. A.; Morales, C. M.; Landis, C. R.; Weinhold, F. Theoretical Chemistry Institute, University of Wisconsin, Madison 2013.
-
[59]
Dapprich S., Frenking G.. Investigation of donor-acceptor interactions: a charge decomposition analysis using fragment molecular orbitals[J]. J. Phys. Chem., 1995, 99: 9352-9362. doi: 10.1021/j100023a009
-
[60]
Krossing I.. Ag(P4)2+: the first homoleptic metal-phosphorus cation[J]. J. Am. Chem. Soc., 2001, 123: 4603-4604. doi: 10.1021/ja002007m
-
[1]
-
Table 1. Front and Side Views of Selected Cluster Anions: a) [Zn (η3-Ge4)2]6-; b) [(η3-E4) Zn (η2-E4)]6- (E = Ge, Sn); c) [Zn{η2-(Si/Ge)4}2]6-, [Au (η2-Sn4)2]7-; d) 1a
Formulas Front view Side view Ref. a) [M(η3-Ge4)2]6- 

18 b) [(η3-E4)M(η2-E4)]6- 19, 25 c) [M(η2-E4)2]n- 24, 30 d) 1a This work Table 2. Relative Energies and Energy Gaps of 1 a Calculated by the B3LYP/ Def2-TZVP Method
Symmetry Relative energy/kJ mol-1 Energy gap/eV Ci 48 2.51 1D2h 92 2.22 C2v 291 1.25 2D2h 0 2.76 -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 5
- 文章访问数: 2208
- HTML全文浏览量: 123


 下载:
下载:


 下载:
下载:

