 Figure 1.
XRD patterns of the hydrothermally synthesized samples at different pH values of the initial solutions: (a) 4, (b) 5, (c) 6, (d) 7, (e) 8, (f) 9, (g) 10, and (h) 12
Figure 1.
XRD patterns of the hydrothermally synthesized samples at different pH values of the initial solutions: (a) 4, (b) 5, (c) 6, (d) 7, (e) 8, (f) 9, (g) 10, and (h) 12

Design of Cadmium Hydroxyapatite Hierarchical Structures with Adjustable Morphology by a Template-free Hydrothermal Route①
English
Design of Cadmium Hydroxyapatite Hierarchical Structures with Adjustable Morphology by a Template-free Hydrothermal Route①
-
Key words:
- template-free
- / hydrothermal
- / cadmium hydroxyapatite
- / hierarchical
-
1 INTRODUCTION
In recent years, the hierarchical structures assembled by nanostructured building blocks (nanoparticles, nanorods, nanobelts, nanosheets, nanowires) have attracted considerable attention due to their unique physical and chemical properties and potential applications in catalysis, medicine, electronics and cosmetics[1-3]. Self-assembly has been shown to be a facile and efficient “bottom-up” route to assembled structures, and up to now many different kinds of materials with hierarchical structures, such as metal[4, 5], metal oxide[6-8], sulfide[9, 10], phosphate[11], hydrate[12] and others[13, 14] have been successfully fabricated by this spontaneous process.
Calcium hydroxyapatite (Ca-Hap, Ca10(PO4)6(OH)2), which is the major constituent of the skeleton and teeth of vertebrates, is tolerant to cationic and anionic substitutions[15, 16]. Various cation-exchanged hydroxyapatites have been achieved through the replacement of divalent ions such as Sr2+, Ba2+, Pb2+, Cu2+ and Cd2+[17]. Among these substituted hydroxyapatites, cadmium hydroxyapatite (Cd-Hap, Cd5(PO4)3(OH)) has been extensively studied during the past century, especially on its very interesting structural properties[18], thermal behavior[19, 20] and spectral characteristics[21]. Many researchers have also focused on the synthesis of Cd-Hap by various synthetic routes. For example, Yasukawa et al.[17] prepared well-crystallized and needle-like Cd-Hap particles via a two-step wet chemical method utilizing acetamide to control the pH of the solution. Zhu et al.[16] reported the hydrothermal formation of Cd-Hap crystals varied from stubby hexagonal prismatic to rod-like in shape. They further investigated the relationship between synthetic conditions and properties of the products such as crystalline phases, crystallinity, and morphology[22]. However, little work has been done on the controlled assembly of low-dimensional building units into complex hierarchical structures of Cd-Hap, and it is necessary to develop a facile route for the synthesis of hierarchical structures and to further investigate their novel properties or applications. More recently, we have reported a simple precipitation method to synthesize hierarchical flowerlike cadmium phosphate microspheres including Cd5H2(PO4)4·4H2O and Cd5(PO4)2P2O7 and investigated their photocatalytic application towards the degradation of dye wastewater under UV light irradiation[23]. Hong and co-workers also reported the application of hydroxyapatite as supports to design Ag3PO4/HA composite photocatalyst to remove pollutant in wastewater under solar light irradiation[24].
Herein, we have successfully synthesized Cd-Hap with several novel morphologies by a facile hydrothermal process without the use of any template or organic surfactant. The typical morphological structures with bunch-, quasi peanut-, and flowerlike crystals can be obtained by simply controlling the pH value of the initial reaction solution. The optical property as well as the photocatalytic activity of pure Cd-Hap and the hydroxyapatite supported Ag3PO4 composites were firstly investigated.
2 EXPERIMENTAL
2.1 Synthesis of the samples
All reagents used in this experiment were of analytical grade without further purification. Hierarchical Cd-Hap crystals were synthesized by a template-free hydrothermal method. In a typical procedure, 0.498 g Cd(CH3COO)2·2H2O and 0.471 g Na3PO4·12H2O were respectively dissolved in 35 mL deionized water with stirring. Then, Na3PO4 solution was added to the Cd(CH3COO)2 solution and the mixture was further stirred for 20 min. The pH value of the solution was adjusted by HNO3 and NaOH solutions. The resulting mixture was then transferred into a 100 mL Teflon-lined stainlesssteel autoclave and kept at different temperature (140 ~ 200 ℃ ) for 1 ~ 12 h in a digital-type temperature-controlled oven and then cooled to room temperature. After hydrothermal reaction, the products were collected, centrifuged, washed with deionized water for several times and finally dried in air at 60 ℃.
The hydroxyapatite supported Ag3PO4 nanoparticles were synthesized by a wet impregnation method. 0.3 g Cd-Hap was firstly dispersed into 20 mL deionized water and the suspension was stirred intensively for 10 min. Then 20 mL 5 mmol/L silver nitrate solution and 20 mL 1.7 mmol/L sodium phosphate were sequentially added into the stirred suspension dropwise. After reacting for 5 h, the suspension was removed from the precipitate by centrifugation and the remaining precipitates were dried at 60 ℃. The products were ground and labeled as AP(5%)/Cd-Hap with a nominal silver phosphate loading of 5 wt%.
2.2 Samples characterization
The crystal phase of the as-synthesized samples was examined by X-ray diffraction (XRD) on a Rigaku MinFlex II equipped with CuKα irradiation (λ = 0.15418 nm). Morphologies of the samples were observed by field emission scanning electron microscope (FESEM, JSM-6700F). The UV-vis reflection spectra (DRS) were recorded on a Varian Cary 500 UV-vis spectrophotometer using BaSO4 as a reference and converted from reflection to absorbance by the Kubelka-Munk method.
Photocatalytic experiments were performed in an aqueous solution at ambient temperature. A 300 W halogen lamp (Philips Electronics) equipped with a composited cut-off filter (400<λ<800 nm) was used as the visible light source. The reaction system was cooled by a fan and circulating water to maintain at room temperature. Briefly, 80 mg of the powder was suspended in 80 mL aqueous solution of methyl orange (MO, 10 ppm). Prior to irradiation, the suspension was magnetically stirred in dark for 0.5 h to establish an adsorption-desorption equilibrium. A 3 mL aliquot was taken at several-minute intervals during the experiment and centrifuged to remove the powders. The residual concentration of dye was analyzed on a Shanghai Youke UV756CRT spectrophotometer. The degradation percentage is reported as C/C0, where C0 is the concentration of initial MO, and C represents the corresponding concentration at a certain time interval.
3 RESULTS AND DISCUSSION
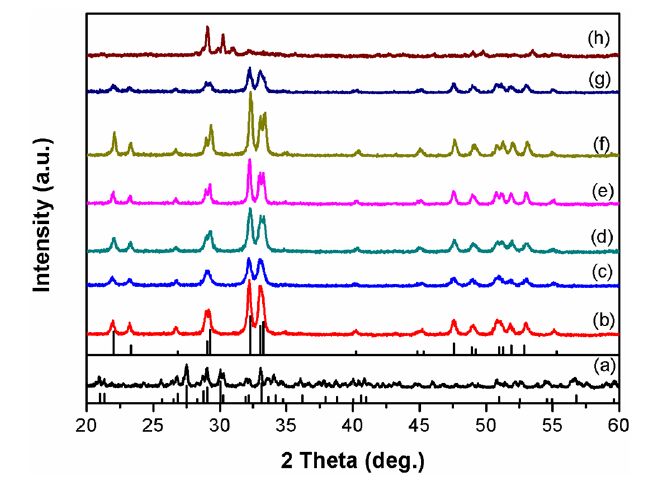
It has been proved that the crystal structure and morphology of Cd-Hap are highly dependent on the pH value of the reaction medium[16, 17, 22]. Thus, in the present synthesis of Cd-Hap crystals, the pH values of the starting solutions before hydrothermal reaction were carefully controlled. Fig. 1 shows the XRD patterns of the hydrothermally synthesized samples at different pH values of the initial solutions. The strong acidic conditions (pH<4) did not favor the formation of solid precipitate until Cd5H2(PO4)4·4H2O (JCPDS no. 14 ~ 0400) was formed at pH of 4. When the pH values of the starting solution were over 5, Cd-Hap was formed as a single phase (JCPDS no. 14~0302). The formation of pure phase of Cd-Hap can be achieved in a wide pH range of 5 ~ 10. However, a small difference in the intensity of the diffraction peaks for series Cd-Hap was observed, indicating the morphological diversity. Further increasing the pH to 12 resulted in the formation of an unknown phase with good crystallinity.
 Figure 1.
XRD patterns of the hydrothermally synthesized samples at different pH values of the initial solutions: (a) 4, (b) 5, (c) 6, (d) 7, (e) 8, (f) 9, (g) 10, and (h) 12
Figure 1.
XRD patterns of the hydrothermally synthesized samples at different pH values of the initial solutions: (a) 4, (b) 5, (c) 6, (d) 7, (e) 8, (f) 9, (g) 10, and (h) 12
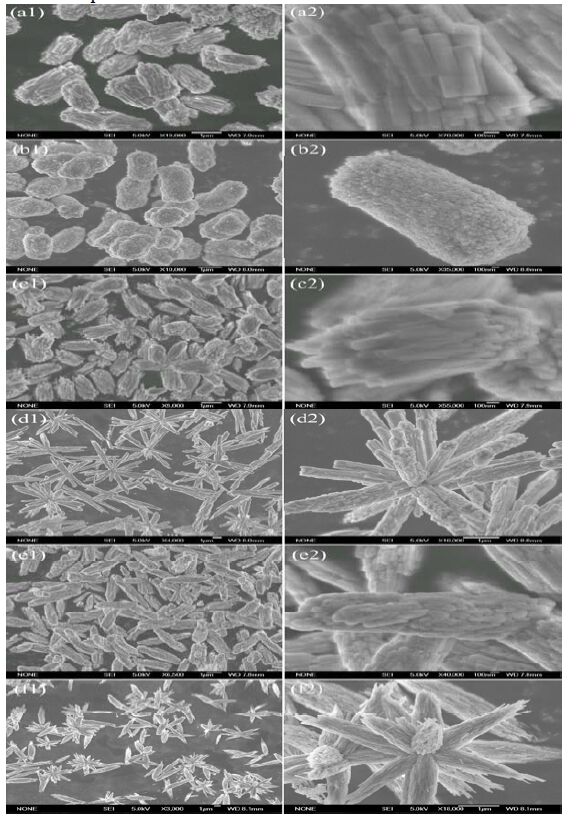
The typical SEM images of the as-synthesized Cd-Hap at different pH values are shown in Fig. 2. Interestingly, various novel Cd-Hap hierarchical structures with a high-yield growth and good sample uniformity were obtained. There are significant differences in the morphology and crystallite sizes of the Cd-Hap hierarchical structures. Cd-Hap crystals prepared at pH 5 were composed of bunch-like hierarchical structures. These bunch-like Cd-Haps were parallelly arranged by nanosized cuboids with an average length of 700 nm and width of 150 nm. When pH value was 6, the Cd-Hap crystals were composed of quasi peanut-like columns that were assembled by numerous nanoparticles with the size of 50 nm. Further increase of the pH to 7 resulted in similar bunch-like structures as that observed at pH 5. However, the length and width of individual cuboids were relatively smaller and the aggregation degree was higher for Cd-Hap at pH 7 than that at pH 5. When the pH was more than 8, complex flower-like architectures were obtained. The flower-like architectures were constructed by small bundles that consisted of smaller nanowires or nanoparticles, which mainly were the structural units obtained at lower pH values (5~7) but with low aspect ratio. As a result, the overall morphology changed from bunch-like hierarchical structures to flower-like architectures when the pH value of the initial solutions was increased from 5 to 10.
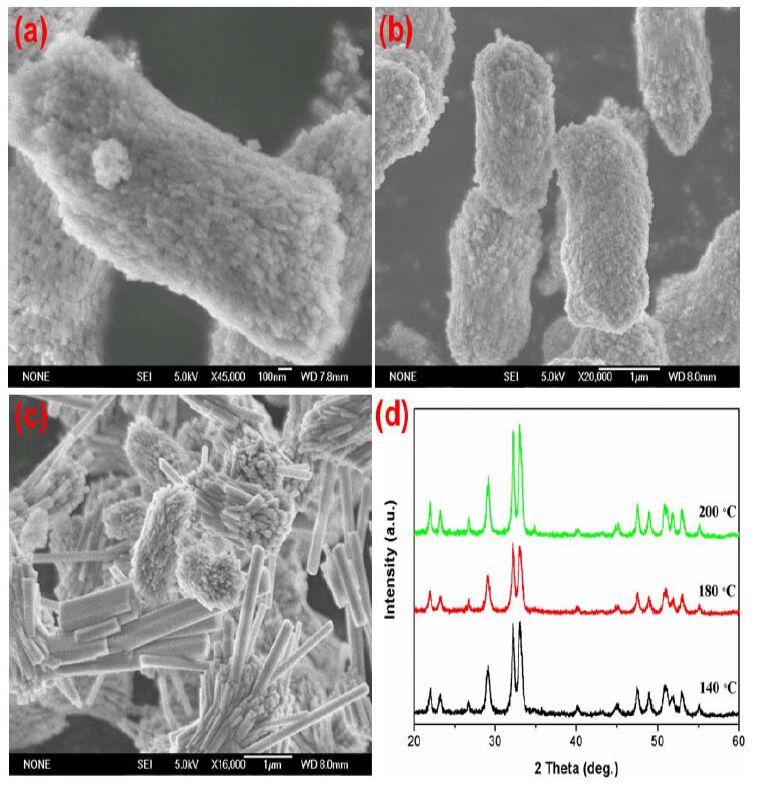
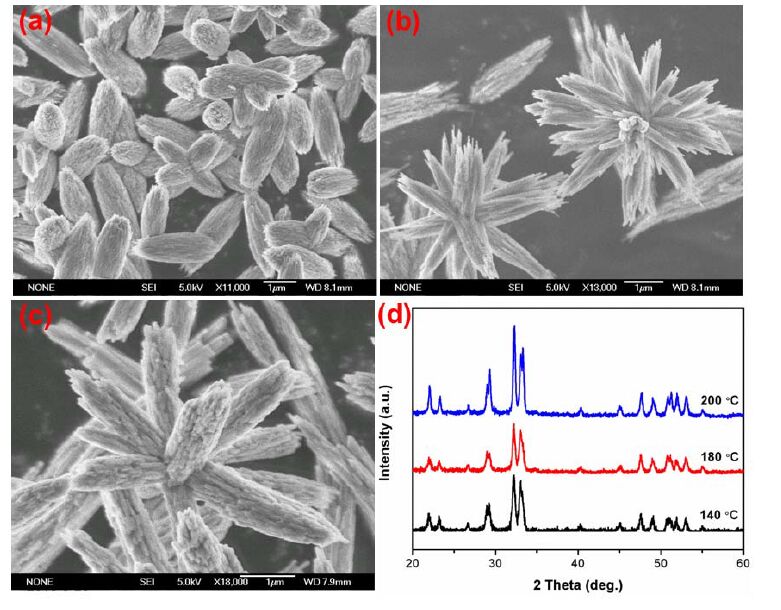
In addition to pH value, the reaction temperature has also been found to play an important role in the formation of Cd-Hap since some intermediate phases such as Cd2P2O7·5H2O are usually formed during hydrothermal process[16]. Thus, we also performed the control experiments to prepare Cd- Hap at different hydrothermal temperature. Fig. 3 illustrates the SEM images and XRD patterns of the products synthesized at different hydrothermal temperature with the initial pH of 6. As can be seen, pure phase of Cd-Hap can be obtained at various temperature. The Cd-Haps prepared at 140 and 180 ℃ were similar quasi peanut-like columns and assembled by nanoparticles. The much higher temperature at 200 ℃ promoted the crystallization and anisotropic growth of Cd-Hap nanoparticles into larger nanorods. Moreover, the elevated hydrothermal temperature could also favor the self-assembly of Cd-Hap crystals. As represented in Fig. 4, when increasing the reaction temperature from 140 to 180 ℃ , the initial bunch-like hierarchical structures changed into flower-like architectures, both of which were composed of numerous nanowires with oriented arrangement. Further increasing the temperature to 200 ℃ had little influence on the morphology of the products but greatly accelerated the growth of the nanowires to short nanorods.
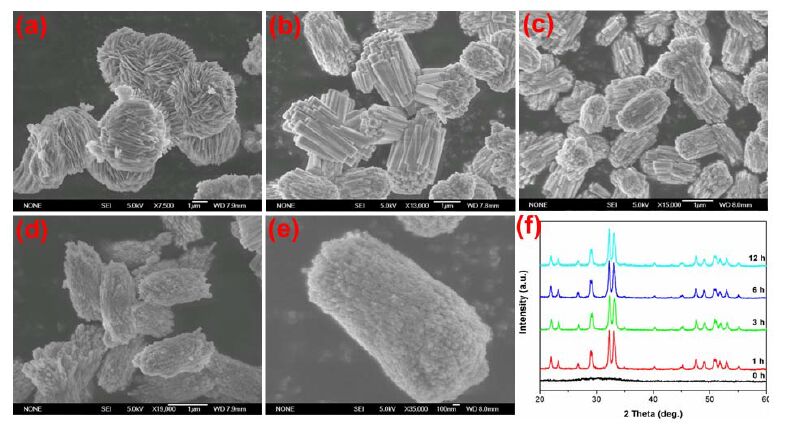
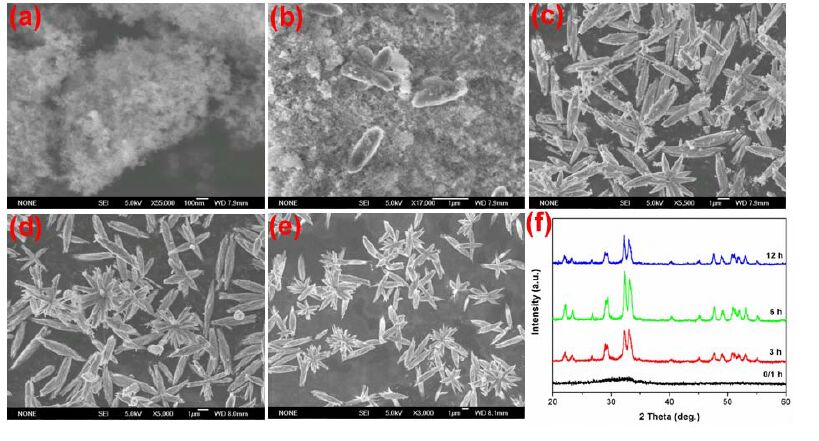
The morphological evolution of the hierarchical Cd-Hap was further investigated by time-dependent experiments. Two typical Cd-Hap structures composed of peanut-like columns and flower-like architectures that were respectively synthesized at pH value of 6 and 10 were selected. For Cd-Hap prepared at pH of 6 (Fig. 5), the precipitate obtained at room temperature without hydrothermal treatment was amorphous Cd5H2(PO4)4·4H2O flower-like microspheres assembled with nanosheets. The formation mechanism of hierarchical Cd5H2(PO4)4·4H2O has been proposed in our previous work[23]. The amorphous phase rapidly changed to well-crystallized Cd-Hap at hydrothermal temperature of 180 ℃ for 1 h. The crystals of Cd-Hap were cuboids in shape and orderly arranged into bunch-like assembly. With increasing the reaction time (3~12 h), the cuboids were gradually slimmed into nanowires and nanoparticles. No obvious change of crystal phase was obtained for Cd-Hap with prolonging the reaction time. In the case of pH 10 (Fig. 6), amorphous ultrafine nanoparticles with size lower than 50 nm were formed. The amorphous phase existed even at 180 ℃ for 1 h and crystallized to Cd-Hap after the reaction time longer than 3 h. During the hydrothermal process, the primary nanoparticles firstly tended to aggregate together to form elliptical- like spheres and then grew into nanowire bundles after 3 h of reaction. The crystallization and assembly of these particles further proceeded and well-defined flower-like architectures were obtained after reaction of 12 h.
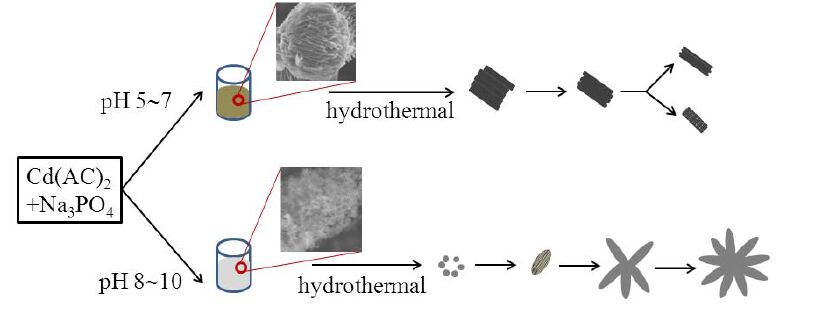
Based on these experimental results and the observation of growth stage, the mechanisms of the formation and evolution of the morphology of Cd-Hap hierarchical structures are proposed in Fig. 7. At low pH of 5~7, the strong acidic environment favored the formation of amorphous Cd5H2(PO4)4·4H2O and preferentially resulted in hierarchical microspheres prior to the hydrothermal process[15, 23]. During the hydrothermal process, more OH− ions were released from the hydrolysis of PO43− and participated in the formation of Cd-Hap by dissolving Cd5H2(PO4)4·4H2O precipitates. Since the cell parameters of both phosphates were very close to each other, the dissolved Cd5H2(PO4)4·4H2O precipitates would act as templates and some Cd- Hap crystallites nucleated on the surface of Cd5H2(PO4)4·4H2O nanosheets finally lead to the formation of assembled bundles like bunch-like and quasi peanut-like structures. On the other hand, when increasing the pH value of the initial solution to 8~10, ultrafine amorphous Cd-Hap nanoparticles were produced at the initial hydrothermal reaction. The freshly formed particles were unstable due to their high surface energy and aggregated together gradually. Meanwhile, the adsorption probability of OH− ions onto the surface of amorphous particles resulted in the anisotropic growth of these particles along the long axis (c-axis) into nanowire bundles, which were further assembled into flower-like architectures[22, 25].
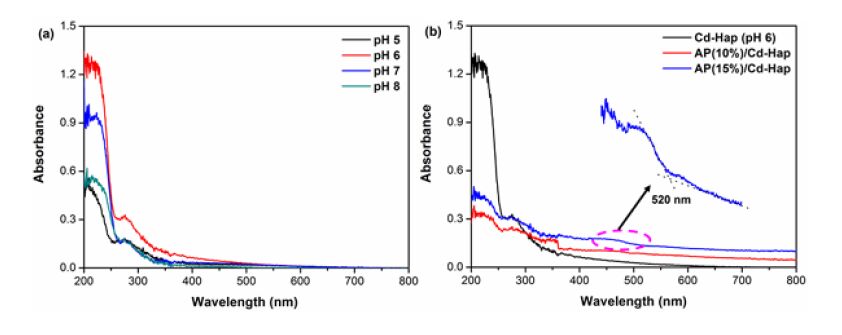
The optical properties of the typical Cd-Hap hierarchical structures as well as the AP/Cd-Hap composites were measured. Fig. 8(a) shows that all the Cd-Hap powders absorb only the UV light with a wavelength shorter than ~350 nm. As compared, the spectra of the AP/Cd-Hap composites appear to be shifted to longer wavelengths in the visible light region (~520 nm) (Fig. 8(b)), which is ascribed to the contribution from Ag3PO4. An apparent enhancement of absorption is observed for the AP/Cd-Hap composites with the increase of Ag3PO4 contents, proving that more Ag3PO4 has been attached onto the surface of Cd-Hap supports. Therefore, AP/Cd-Hap composites inherently possess the photocatalytic potential for the uphill reactions involved in organic pollutant degradation under visible light irradiation.
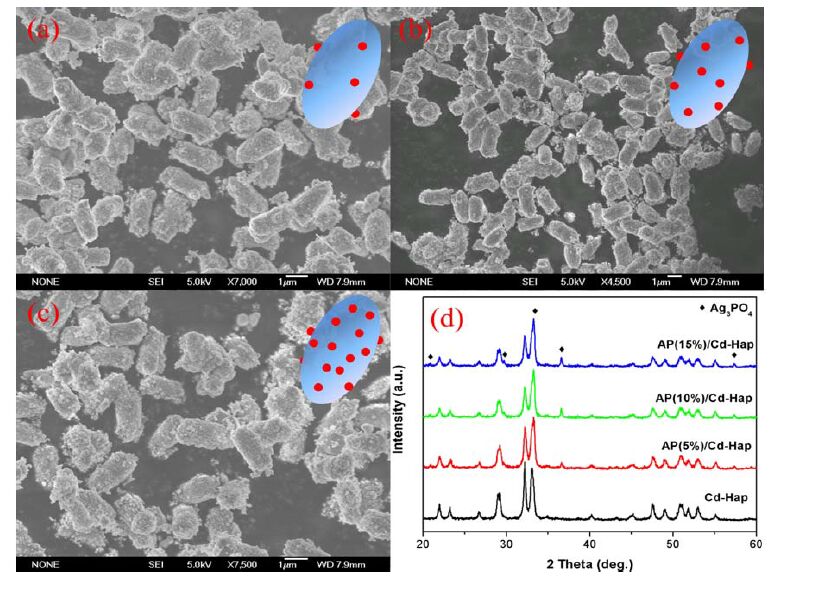
The representative morphology of AP/Cd-Hap composites was revealed by SEM measurements and the results are shown in Fig. 9. It can be seen that highly dispersed Ag3PO4 nanoparticles with average size of 100 nm have been deposited onto the surfaces of Cd-Hap supports, which remain the original peanut-like morphology. The larger silver ion loading resulted in higher Ag3PO4 nanoparticle density on a unit volume basis of Cd-Hap supports. The formation of cubic Ag3PO4 in AP/Cd-Hap composites can also be proved by the typical XRD patterns in Fig. 9(d).
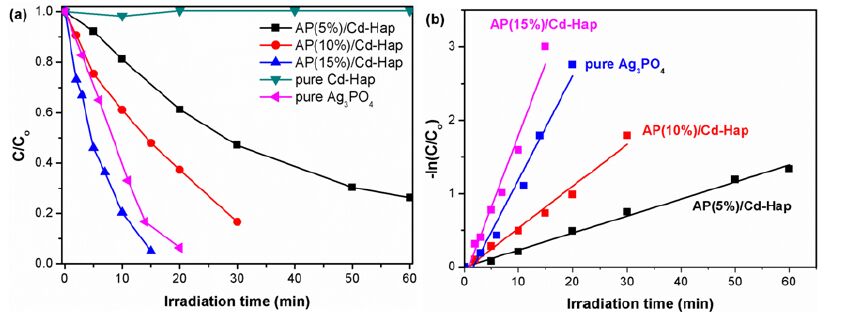
The photocatalytic activity of Cd-Hap supports and AP/Cd-Hap composites was evaluated by degradation of methyl orange (MO) in aqueous solution under visible light irradiation. There was no obvious change of MO concentration on pure Cd-Hap under visible light irradiation in 60 min. However, AP/Cd-Hap composites showed remarkable photocatalytic activity and the activity was strongly dependent on the Ag3PO4 contents (Fig. 10(a)). With the Ag3PO4 contents increasing, the photocatalytic activities of the composites were gradually enhanced. 15% AP/Cd-Hap showed the highest degradation efficiency. It is noted that MO was almost degraded after being irradiated for 15 min over AP(15%)/Cd-Hap composite. As compared, the photocatalytic activity of pure Ag3PO4 was only at 85% after irradiation for 15 min, suggesting that AP/Cd-Hap composites containing a low content of active Ag3PO4 (only 15 %) have better performance in the photodegradation of MO than pure Ag3PO4. Although further increasing Ag3PO4 content in AP/Cd-Hap composite would benefit the degradation of MO, the high agglomeration of more Ag3PO4 particles on Cd-Hap surface and the increasing cost of expensive Ag3PO4 restrict the practical application of AP/Cd-Hap with high Ag3PO4 content. It has also been found that the photocatalytic degradation of MO followed the pseudo-first-order kinetics (Fig. 10(b)). The degradation rate over AP(15%)/Cd-Hap composite was estimated to be 0.19484 min-1, which is approximately 1.37 times of pure Ag3PO4 (0.14158 min-1). The improved photocatalytic performance of AP(15%)/Cd-Hap can mainly be attributed to the high dispersion of Ag3PO4 nanoparticles over the Cd-Hap supports. Such a high dispersion suggests much of the reactive surface of the Ag3PO4 particles located within the composites, which may greatly improve the ratio of the surface charge carrier transfer rate to the electron-hole recombination rate, the adsorption of organic pollutant or the visible light absorbance property and thus the photocatalytic activities[23].
4 CONCLUSION
In summary, a facial hydrothermal route to hierarchical cadmium hydroxyapatite (Cd-Hap) with novel superstructures without employing surfactant or templates is found. The results show that the pH value and reaction temperature possess significant influences on the assembly and growth process and further affect both the morphology of superstructures and that of the building units. The optical measurement demonstrates that the as-obtained Cd-Hap can only absorb UV light with wavelength shorter than 350 nm. However, the hydroxyapatite supported Ag3PO4 composites show excellent photocatalytic activity toward the degradation of MO under visible light irradiation, even superior to that of the pure Ag3PO4 photocatalyst.
-
-
[1]
Kim F., Kwan S., Akana J., Yang P. D.. Langmuir-blodgett nanorod assembly[J]. J. Am. Chem. Soc., 2001, 123: 4360-4361. doi: 10.1021/ja0059138
-
[2]
An Z. G., Zhang J. J., Pan S. L., Yu F.. Facile template-free synthesis and characterization of elliptic α-Fe2O3 superstructures[J]. J. Phys. Chem. C, 2009, 113: 8092-8096. doi: 10.1021/jp9004168
-
[3]
Li Z. Q., Ding Y., Xiong Y. J., Yang Q., Xie Y.. One-step solution-based catalytic route to fabricate novel alpha-MnO2 hierarchical structures on a large scale[J]. Chem. Commun., 2005, 7: 918-920.
-
[4]
Lopes W. A., Jaeger H. M.. Hierarchical self-assembly of metal nanostructures on diblock copolymer scaffolds[J]. Nature, 2001, 414: 735-738. doi: 10.1038/414735a
-
[5]
Chen J. Y., Wiley B. J., Xia Y. N.. One-dimensional nanostructures of metals: large-scale synthesis and some potential applications[J]. Langmuir, 2007, 23: 4120-4129. doi: 10.1021/la063193y
-
[6]
Zhang L., Wu H. B., Madhavi S., Hng H. H., Lou X. W.. Formation of Fe2O3 microboxes with hierarchical shell structures from metal-organic frameworks and their lithium storage properties[J]. J. Am. Chem. Soc., 2012, 134: 17388-17391. doi: 10.1021/ja307475c
-
[7]
Blin J. L., Léonard A., Yuan Z. Y., Gigot L., Vantomme A., Cheetham A. K., Su B. L.. Hierarchically mesoporous/macroporous metal oxides templated from polyethylene oxide surfactant assemblies[J]. Angew. Chem., 2003, 115: 2978-2981. doi: 10.1002/ange.200250816
-
[8]
Yang P. D., Deng T., Zhao D. Y., Feng P. Y., Pine D., Chmelka B. F., Whitesides G. M., Stucky G. D.. Hierarchically ordered oxides[J]. Science, 1998, 282: 2244-2246. doi: 10.1126/science.282.5397.2244
-
[9]
Zhao Q. R., Xie Y., Zhang Z. G., Bai X.. Size-selective synthesis of zinc sulfide hierarchical structures and their photocatalytic activity[J]. Cryst. Growth Des., 2007, 7: 153-158. doi: 10.1021/cg060521j
-
[10]
Zhu T., Wang Z. Y., Ding S. J., Chen J. S., Lou X. W.. Hierarchical nickel sulfide hollow spheres for high performance supercapacitors[J]. RSC Adv., 2011, 1: 397-400. doi: 10.1039/c1ra00240f
-
[11]
Gutiérrez M. C., Jobbágy M., Ferrer M. L., Monte F. D.. Enzymatic synthesis of amorphous calcium phosphate-chitosan nanocomposites and their processing into hierarchical structures[J]. Chem. Mater., 2008, 20: 11-13. doi: 10.1021/cm7020164
-
[12]
Wang D. B., Song C. X., Hu Z. S., Fu X.. Fabrication of hollow spheres and thin films of nickel hydroxide and nickel oxide with hierarchical structures[J]. J. Phys. Chem. B, 2005, 109: 1125-1129.
-
[13]
Yu S. H., Antonietti M., C?lfen H., Hartmann J.. Growth and self-assembly of BaCrO4 and BaSO4 nanofibers toward hierarchical and repetitive superstructures by polymer-controlled mineralization reactions[J]. Nano. Letters, 2003, 3: 379-382. doi: 10.1021/nl025722y
-
[14]
Whang D., Jin S., Wu Y., Lieber C. M.. Large-scale hierarchical organization of nanowire arrays for integrated nanosystems[J]. Nano. Letters, 2003, 3: 1255-1259. doi: 10.1021/nl0345062
-
[15]
Arami H., Mazloumi M., Khalifehzadeh R., Lak A., Sadrnezhaad S. K.. Self-assembled dahlia-like cadmium hydrogen phosphate hydrate nanostructures as templates for cadmium hydroxyapatite hexagonal prisms[J]. J. Crystal Growth, 2007, 309: 37-42. doi: 10.1016/j.jcrysgro.2007.08.021
-
[16]
Zhu K. J., Yanagisawa K., Onda A., Kajiyoshi K.. Hydrothermal synthesis and morphology variation of cadmium hydroxyapatite[J]. J. Solid State Chem., 2004, 177: 4379-4385. doi: 10.1016/j.jssc.2004.09.025
-
[17]
Yasukawa A., Yokoyama T., Ishikawa T.. Preparation of cadmium hydroxyapatite particles using acetamide[J]. Mater. Res. Bull., 2001, 36: 775-786. doi: 10.1016/S0025-5408(01)00536-0
-
[18]
Hata M., Okada K., Iwai S., Akao M., Aoki H.. Cadmium hydroxyapatite[J]. Acta Crystallogr. B, 1978, 34: 3062-3064. doi: 10.1107/S0567740878010031
-
[19]
Ropp R. C., Mooney R. W.. Phosphates of cadmium[J]. J. Am. Chem. Soc., 1960, 82: 4848-4852. doi: 10.1021/ja01503a025
-
[20]
Ropp R. C., Mooney R. W., Hoffman C. W. W.. X-ray powder diffraction patterns of some cadmium phosphates[J]. Anal. Chem., 1961, 33: 1687-1689. doi: 10.1021/ac60180a021
-
[21]
Engel G., Klee W. E.. Infrared spectra of the hydroxyl ions in various apatites[J]. J. Solid State Chem., 1972, 5: 28-34. doi: 10.1016/0022-4596(72)90004-7
-
[22]
Zhu K. J., Yanagisawa K., Onda A., Kajiyoshi K., Qiu J. H.. Morphology variation of cadmium hydroxyapatite synthesized by high temperature mixing method under hydrothermal conditions[J]. Mater. Chem. Phys., 2009, 113: 239-243. doi: 10.1016/j.matchemphys.2008.07.049
-
[23]
Yan T. J., Guan W. F., Cui L. T., Xu Y. Q., Tian J.. Immobilization of cadmium ions to synthesis hierarchical flowerlike cadmium phosphates microspheres and their application in the degradation of organic pollutants under light irradiation[J]. RSC Adv., 2015, 5: 43756-43764. doi: 10.1039/C5RA07224G
-
[24]
Hong X. T., Wu X. H., Zhang Q. Y., Xiao M. F., Yang G. L., Qiu M. R., Han G. C.. Hydroxyapatite supported Ag3PO4 nanoparticles with higher visible light photocatalytic activity[J]. Appl. Surf. Sci., 2012, 258: 4801-4805. doi: 10.1016/j.apsusc.2012.01.102
-
[25]
Cho I. S., Kim D. W., Lee S., Kwak C. H., Bae S. T., Noh J. H., Yoon S. H., Jung H. S., Kim D. W., Hong K. S.. Synthesis of Cu2PO4OH hierarchical superstructures with photocatalytic activity in visible light[J]. Adv. Funct. Mater., 2008, 18: 2154-2162. doi: 10.1002/adfm.v18:15
-
[1]
-
-

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 2
- 文章访问数: 1669
- HTML全文浏览量: 143


 下载:
下载:









 下载:
下载:

