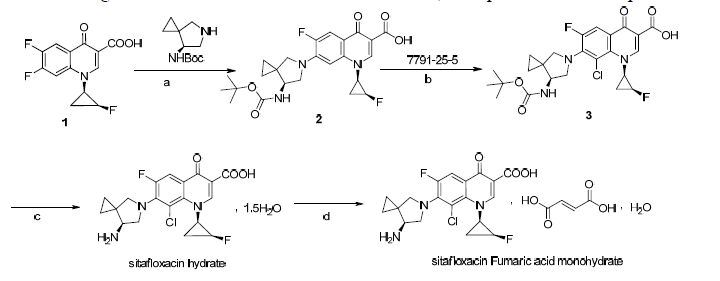
 Figure Scheme1.
Procedure for synthesis of the title compound I
Figure Scheme1.
Procedure for synthesis of the title compound I

Synthesis and Crystal Structure of 7-((7S)-7-Aminospiro[2.4]heptan-5-yl)-8-chloro-6-fluoro-1-((1R,2S)-cis-2-fluorocyclopropyl)-4-oxo-1,4-dihydroqun-oline-3-carboxylic Acid Fumaric Acid Monohydra①
English
Synthesis and Crystal Structure of 7-((7S)-7-Aminospiro[2.4]heptan-5-yl)-8-chloro-6-fluoro-1-((1R,2S)-cis-2-fluorocyclopropyl)-4-oxo-1,4-dihydroqun-oline-3-carboxylic Acid Fumaric Acid Monohydra①
-
Key words:
- sitafloxacin
- / synthesis
- / crystal structure
-
1 INTRODUCTION
Sitafloxacin is a new-generation oral fluoroquinolone developed by Daiichi Sankyo Co., Ltd. with the name of Gracevit. Sitafloxacin has been proved to exhibit high and broad-spectrum antibacterial activity against Gram-positive and Gram-negative aerobes and anaerobes, a variety of clinical common fluoroquinolone resistance bacteria, mycoplasma and chlamydia[1, 2]. Sitafloxacin monohydrates have been clinically used. However, as a weak basic complex, sitafloxacin shows a pH dependent dissolution characteristics and is lack of light stability in aqueous solution[3]. In addition, after its injection into body, sitafloxacin can produce certain stimulating effects and increase the drug side-effects and risks. In order to further optimize the medicinal properties of sitafloxacin, sitafloxacin salt compounds have been synthesized by Chao et al., which exhibit different degree of improvement of medicinal properties. Furthermore, fumarate displays the following advantages: the solubility in water was 10 times bigger than the free base under the fumarate (1.6 mg/mL) vs. the free base (0.11 mg/mL); the stability was significantly increased at 60 ℃ for 14 days with its impurities and crystal shape unchanged, while free alkali impurities increased by 0.06% and crystal type showed obvious variation. Moreover, in sharp test, the skin tissue did not show obvious swelling, blood stasis erosion or ulcer lesions in the sitafloxacin fumarate group pushing injection parts, whereas obvious swelling was observed in the skin tissue with free alkali group pushing injection and swelling phenomenon still lasted after the drug was stopped for 48 hours, suggesting that the stimulus of sitafloxacin fumarate is lower than the sitafloxacin. In this study, the structural properties of a sitafloxacin fumaric monohydrate have been acquired by single-crystal XRD and further characterized by 1H NMR, 14C spectra and mass spectra.
The procedure for I is shown in Scheme 1. 7-[7- (S)-bocaminospiro[2.4]heptan-5-yl)-6-fluoro-1-((1R, 2S)-2-fluorocyclopr-opyl)-4-oxo-1, 4-dihydroquinoline- 3-carboxylica (compound 2) was prepared by the condensation reaction of 6, 7-difluoro-c-((1R, 2S)-2- fluorocyclopr-opyl)-4-oxo-1, 4-dihydroquinoline-3- carboxylica (compound 1) and ((S)-7-bocaminospiro[2.4]heptan under alkaline conditions; 7-[7- (S)-bocaminospiro[2.4]heptan-5-yl)-8-chloro-6-fluo ro-1-((1R, 2S)-2-fluorocyclopropyl)-4-oxo-1, 4-dihyd roquinoline-3-carboxylic (compound 3) is obtained by the substitution reaction of compound 2 and sulfonyl chloride; Compound 3 turns into the sitafloxacin hydrate under the condition of acid protection[6]; Sitafloxacin fumaric acid monohydrate is synthesized with the sitafloxacin hydrate and fumaric acid in the ethanol/ethyl acetate solvent.
2 EXPERIMENTAL
2.1 Materials and measurements
All reagents were purchased commercially and used without further purification. 1H NMR and 14C spectra were recorded on a Bruker AV-400 NMR spectrometer using TMS as an internal reference in CDCl3/DMSO-d6 solution. Mass spectra were performed by an Agilent G1956B single-quadrupole instrument using ESI ionization method in the methanol aqueous solution (80:20, V/V).
2.2 Synthesis of the title compound (I)
Preparation of 7-[7-(S)-bocaminospiro[2.4]heptan- 5-yl)-6-fluoro-1-((1R, 2S)-2-fluorocyclopropyl)- 4-oxo-1, 4-dihy-droquinoline-3-carboxylic (2): A mixture of 1 (283.0 mg, 1.0 mmol), (S)-7-bocaminospiro[2.4]heptan (213.0 mg, 1.0 mmol), (C2H5)3N (1.0 mL) and CH3CN (20 mL) was refluxed for 3.0 h under N2 atmosphere in a 100 mL eggplant bottle. The reaction mixture was handled by concentration, sand and column chromatograph (CH2Cl2: CH3OH: HAc = 20:1:1), obtianing white floccular solid (438.0 mg) in yield 90%. 1H NMR (400 MHz, DMSO-d6) δ: 8.65 (s, 1H), 7.82 (d, J = 14.0, 1H), 7.36 (d, J = 7.2, 1H), 6.95 (d, J = 7.6, 1H), 5.44 (d, J = 64.4, 1H), 3.82~3.98 (m, 4H), 3.63 (d, J = 10.8, 1H), 3.41 (d, J = 8.0, 1H), 1.93 (d, J = 6.0, 1H), 1.76~1.79 (m, 1H), 1.39 (s, 9H), 0.86 (br, 1H), 0.70~0.72 (m, 3H). HPLC: 98.79%. ESI-MS: 476.5 [M+H]+.
Preparation of 7-[7-(S)-bocaminospiro[2.4]heptan- 5-yl)-8-chloro-6-fluoro-1-((1R, 2S)-2-fluorocyclopropyl)- 4-oxo-1, 4-dihydroquinoline-3-carboxylic (3): compound 2 (238 mg, 0.5 mmol) was dissolved in CH2Cl2 (30 mL) in a 100 mL three-hole bottle and cooled to 0~5 ℃ with stirring; the solution of sulfony chloride (119 mg, 1 mmol) in 10 mL CH2Cl2 was dropwise added to the above solution and stirred for 0.5 h under 0~5 ℃. Then 20 mL ice water was added and alkalized till pH = 7~8. The mixture was extracted with CH2Cl2 (100 mL × 3). The organic phase was dried with anhydrous MgSO4. After column chromatograph (ethylacetate:petroleum ether = 6:1), the white powder solid (221.5 mg) was obtained in 87% yield. 1H NMR (400 MHz, DMSO-d6) δ: 14.68 (s, 1H), 8.77 (d, J = 3.2, 1H), 7.84 (d, J = 14.0, 1H), 7.24 (d, J = 6.8, 1H), 5.21 (d, J = 64.0, 1H), 4.27~4.31 (m, 2H), 4.22 (d, J = 5.2, 13.6, 1H), 3.77 (br, 1H), 3.43 (d, J = 10.8, 1H), 3.25 (d, J = 10.0, 1H), 1.61~1.64 (m, 1H), 1.39 (s, 9H), 0.84~0.87 (m, 1H), 0.60~0.70(m, 3H). HPLC: 98.77%. ESI-MS: 510.9 [M+H]+.
Preparation of 7-((7S)-7-aminospiro[2.4]heptan- 5-yl)-8-chloro-6-fluoro-1-((1R, 2S)-2-fluorocyclo propyl)-4-oxo-1, 4-dihydroquinoline-3-carboxylic acid (sitafloxacin hydrate) A mixture of 2 (510 mg, 1 mmol, trifluoroacetic acid (TFA, 2.4 mL), benzaldehyde (0.8 mL) and CH2Cl2 (10 mL) was stirred and cooled to 0~5 ℃ for 2 h in a 50 mL eggplant bottle, then the reaction mixture was handled by concentration. 10 mL HCl (18%) was added to the above solution and extracted with CH2Cl2 (100mL × 3). The water phase was alkalized with saturated sodium carbonate to pH = 6.5 and filtrated, and the white solid (355.1mg) was collected in the yield of 85%. 1H NMR (400 MHz, DMSO-d6) δ: 8.77 (d, J = 3.3, 1H), 8.24 (m, 1H), 7.90 (d, J = 13.4, 1H), 5.13 (d, J = 63.9, 1H), 4.33~ 4.47 (m, 3H), 3.72 (d, J = 11.6, 1H), 3.54 (m, 1H), 3.23 (d, J = 10.0, 1H), 1.40~1.66 (m, 2H), 0.84~ 1.20 (m, 4H). HPLC: 99.37%. ESI-MS: 410.0 [M+H]+; 432.0 [M+Na]+.
Preparation of 7-((7S)-7-aminospiro[2.4]heptan- 5-yl)-8-chloro-6-fluoro-1-((1R, 2S)-2-fluorocyclo- propyl)-4-oxo-1, 4-dihydroquinoline-3-carboxyli c acid fumaric acid monohydrate (I) A mixture of sitafloxacin hydrate (427 mg, 1 mmol), fumaric acid (116 mg, 1 mmol), EtOH (15 mL) and ethylacetate (1.5 mL) was stirred with 40~50 ℃ under reflux for 1 h in a 50 mL eggplant bottle. Then the recation mixture was filtrated, obtaining white crystals (462 mg) in 85% yield. 1H NMR(400 MHz, DMSO-d6) δ: 8.78 (d, J = 3.6, 1H), 7.87 (d, J = 14.0, 1H), 6.45 (s, 2H), 5.20 (d, J = 58.8, 1H), 4.26~4.37 (m, 3H), 3.54 (d, J = 11.2, 1H), 3.32~3.34 (m, 1H), 3.24 (d, J = 9.2, 1H), 1.39~1.62 (m, 2H), 1.00 (d, J = 10.0, 1H), 0.69 ~ 0.79 (m, 3H). 13C NMR(100MHz, DMSO-d6) δ: 176.18, 168.31(d, J = 6.0), 165.64, 155.12 (d, J = 251.0), 154.15, 142.47 (d, J = 12.0), 139.26, 135.44, 120.46 (d, J = 32.0), 112.33 (d, J = 7.0), 110.59 (d, J = 24.0), 108.14, 74.96 (d, J = 223.0), 58.01, 57.08 (d, J = 9.0), 55.86, 41.90 (d, J = 8.0), 25.83, 16.27(d, J = 10.0), 13.6, 5.48. HPLC: 99.57%. ESI-MS: 410.9 [M+H]+.
2.3 Crystal structure determination
Block crystals of I for X-ray analysis were obtained by vapour diffusion from EtOH/ethylacetate (100:1) in a 100 mL conical flack at room temperature. A single crystal with dimensions of 0.2mm × 0.2mm × 0.2mm was mounted on an Enraf-Nonius CAD4 Express with graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å)[7-11]. The intensity data were collected by using an ω-2θ scan mode in the range of 1.8<θ <30.0° at 293 ± 1 K. A total of 6886 reflections were obtained and 6508 were observed. The structure was solved by direct methods with SHELEXTS-97 and expanded using Fourier difference technique. The non-H atoms were refined anisotropically, and the hydrogen atoms were added theoretically. The final cycle of full-matrix least-squares refinement was based on the observed reflections and variable parameters. The final refinement gave R = 0.0307, wR = 0.0892 (w = 1/[σ2(Fo 2) + (0.0584P)2 + 0.0000P], where P = (Fo 2 + 2Fc 2)/3), S = 1.099, (Δρ)max = 0.30 and (Δρ)min = -0.25 e/Å3. The selected bond lengths, bond angles, torsion angles and H-bonds for I are listed in Tables 1~3, respectively.
Bond Dist. Bond Dist. Bond Dist. C(1)-C(2) 1.485(2) C(2)-C(3) 1.428(2) C(3)-C(4) 1.451(2) C(4)-C(5) 1.406(2) C(2)-C(6) 1.367(2) C(5)-C(10) 1.414(2) C(10)-C(11) 1.410(2) C(4)-C(13) 1.401(2) C(11)-C(12) 1.426(2) C(12)-C(13) 1.360(2) C(5)-N(1) 1.399(2) C(6)-N(1) 1.348(2) C(11)-N(2) 1.379(2) C(1)-O(1) 1.326(2) C(3)-O(3) 1.261(2) Angle (°) Angle (°) Angle (°) C(1)-C(2)-C(3) 122.07(1) C(3)-C(4)-C(13) 119.28(1) C(7)-C(8)-C(9) 58.67(1) C(15)-C(16)-C(18) 117.92(1) C(18)—C(19)—C(16) 60.96(1) C(6)-N(1)-C(7) 116.05(1) C(10)-C(11)-N(2) 122.52(1) N(2)-C(14) -C(15) 103.40(1) O(1)-C(1)-C(2)-C(6) 5.1(1) C(2)-C(6)-N(1)-C(7) -160.66(1) N(1)-C(5)-C(10)-C(11) -20.97(1) C(11)-N(2)-C(14)-C(15) -179.47(1) C( 16)-C( 18)-C(19)-C( 17) -111.3(1) N(2)-C(14)-C(15)-N(3) -83.65(1) C(6)-C(2)-C(3)-O(3) 173.48(1) Table 1. Selected Bond Lengths (Å), Bond Angles (°) and Torsion Angles (°)D-H…A d(D-H) d (H…A) d (D-A) ZD-H…A N(3)…O(8)i 0.91 1.97 2.843(2) 160.3 N(3)-H(3B)…O(4)ii 0.91 2.26 3.024(2) 141.7 N(3)—H(3B)…O(6)iii 0.91 2.40 2.996(2) 122.9 N(3)-H(3B)-Cl(1)iv 0.91 2.90 3.442(2) 119.7 N(3)-H(3C)…O(7)v 0.91 1.90 2.745(2) 154.5 O(1)-H(1)-O(3) 0.84 1.76 2.538(2) 153.4 O(5)-H(5)…O(6)v1 0.84 1.67 2.508(2) 172.2 O(8)-H(8B)-O(7)v 0.82(2) 1.98(2) 2.793(2) 171(3) O(8)-H(8B)-O(6)v 0.82(2) 2.63(2) 3.239(2) 132(3) O(8)-H(8A)-O(4)11 0.80(2) 2.56(2) 3.249(2) 145(3) Symmetry codes: (i): –x+2, y+1/2, –z; (ii): –x+1, y–1/2, –z+1; (iii): x, y, z–1; (iv): –x+2, y–1/2, –z; (v): –x+2, y–1/2, –z+1; (vi): –x+1, y+1/2, –z+2 Table 2. Hydrogen Bond Geometry (Å, °) of Compound IH-Cg X-H …Cg X-Cg C(18)-H(18B)…Cg(4)#1 2.83 150.0 3.719(2) Symmetry codes: #1: 2–x, –1/2+y, 1–z. Ring Cg(4)#1: N(1)→C(5)→C(4)→C(3)→C(2)→C(6)→ Table 3. C-H···π Interactions for Compound I (Å, °)3 RESULTS AND DISCUSSION
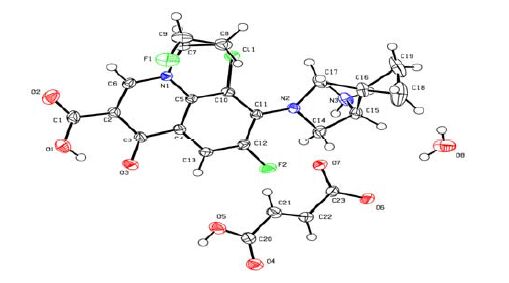
As shown in Table 1 and Fig. 1, the bond lengths of C(2)-C(6), C(4)-C(5), C(10)-C(11) and C(3)- O(3) are obviously longer than those of normal C=C (1.34 Å) and C=O (1.23 Å). However, the relevant bond lengths of C(5)-N(1), C(6)-N(1), C(11)-N(2), C(2)-C(3), C(3)-C(4), C(1)-C(2), C(5)-C(10), C(11)-C(12), C(4)-C(13), C(12)-C(13) and C(1)- O(1) are shorter than those of the normal C(sp2)-N (1.43 Å), C-C (1.53 Å) and C(sp2)-O (1.53 Å). These bond features verfiy its certain delocalization degree, which suggests pyridyl ring, benzene ring and carbonyl group form a big conjugated system.
All atoms of quinoline (N(1)/C(5)/C(10)/ C(11)/ C(12)/C(13)/C(4)/C(3)/C(2)/C(6)) exhibit good coplanar nature, where N(5) and C(10) show the deviations of -0.121(1) and 0.119(1) Å, respectively. The pyrrole ring (N(2)/C(14)/C(15)/C(16)/C(17)) adopts an envelope conformation (φ = 70.1(3)°) with puckering amplitude of 0.366(2) Å.
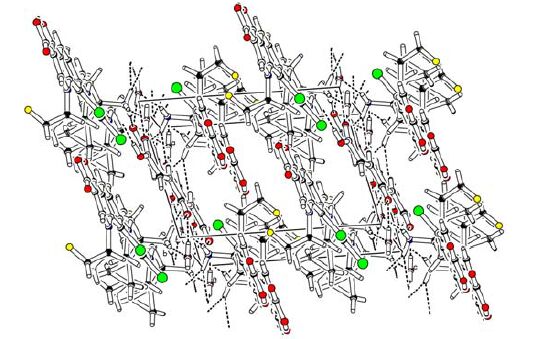
Fig. 2 shows packing diagram of I with hydrogen bonding and C···π interactions. The intramolecular and intermolecular hydrogen bonds (N-H···O, O-H···O and N-H···Cl) connect the molecules into a one-dimensional chain along the b axis. Meanwhile, the sub-H(18B) of pyrrole ring is close to (2.83 Å) the centre (Cg4) of the adjacent pyrrole molecule (N(1)/C(5)/C(4)/C(3)/C(2)/C(6)), which suggests the absence of C-H···π in the molecules. Furthermore, hydrogen bonding together with C···π interactions contribute to the formation and stabi- lization of the three-dimensional framework.
-
-
[1]
Keating. G. M.. Sitafloxacin: in bacterial infections[J]. Drugs, 2011, 71: 731-744. doi: 10.2165/11207380-000000000-00000
-
[2]
Anderson. D. L.. Sitafloxacin hydrate for bacterial infections[J]. Drugs Today (Barc)., 2008, 44: 489-501. doi: 10.1358/dot.2008.44.7.1219561
-
[3]
Araki, M.; Matsukawa, A.; Nakagami, H. Stable liquid preparation. WO2001080858A1 2001.
-
[4]
Chao. Y., Ye. H., Che. X. M.. Sitafloxacin fumarate salt A and pharmaceutical purposes[J]. China patent, 2012, 1006089: .
-
[5]
Zhang. F., Xu. X. X., Shi. W. L.. Sitafloxacin fumarate salt A and pharmaceutical purposes[J]. China patent, 2012, 10201778: .
-
[6]
Zgang. H. B., Miu. S. F., Cheng. L. W.. Synthesis research of sitafloxacin[J]. J. Strait Pharm., 2014, 26: 155-158.
-
[7]
Bukovskii, M. I.; Solodushenkov, S. N.; Mosiichuk, A. I.; Kukhar, V. P. (Phosphoranylideneamino)-s-triazines Ⅲ. Oxidative imination of tervalent phosphorus compounds with azido-s-triazines. Translated from ZhurnalObshcheiKhimii. 1970, 40, 782−784.
-
[8]
Wu. Z. Y., Xu. W., Xia. J. K., Liu. Y. C., Wu. Q. X., Xu. W. J.. Flame retardant polyamide 6 by in situ polymerization of-caprolactam in the presence of melamine derivatives[J]. Chin. Chem. Lett., 2008, 19: 241-244. doi: 10.1016/j.cclet.2007.12.012
-
[9]
Borkovec. A. B., Demilo. A. B.. Insect chemosterilants. Ⅴ. Derivatives of melamine[J]. Insect Chemosterilants, 1967, 10: 457-461.
-
[10]
Saplinova. T., Lehnert. C., Bohme. U., Waglera. J., Kroke. E.. Melem- and melamine-derived iminophosphoranes[J]. New J. Chem., 2010, 34: 1893-1908. doi: 10.1039/b9nj00621d
-
[11]
X-ray Single-crystal Analysis System, Version 2.2, Bruker AXS Inc., 6300 Enterprise Lane, Madison, WI 53719−1173, USA.
-
[1]
-
Table 1. Selected Bond Lengths (Å), Bond Angles (°) and Torsion Angles (°)
Bond Dist. Bond Dist. Bond Dist. C(1)-C(2) 1.485(2) C(2)-C(3) 1.428(2) C(3)-C(4) 1.451(2) C(4)-C(5) 1.406(2) C(2)-C(6) 1.367(2) C(5)-C(10) 1.414(2) C(10)-C(11) 1.410(2) C(4)-C(13) 1.401(2) C(11)-C(12) 1.426(2) C(12)-C(13) 1.360(2) C(5)-N(1) 1.399(2) C(6)-N(1) 1.348(2) C(11)-N(2) 1.379(2) C(1)-O(1) 1.326(2) C(3)-O(3) 1.261(2) Angle (°) Angle (°) Angle (°) C(1)-C(2)-C(3) 122.07(1) C(3)-C(4)-C(13) 119.28(1) C(7)-C(8)-C(9) 58.67(1) C(15)-C(16)-C(18) 117.92(1) C(18)—C(19)—C(16) 60.96(1) C(6)-N(1)-C(7) 116.05(1) C(10)-C(11)-N(2) 122.52(1) N(2)-C(14) -C(15) 103.40(1) O(1)-C(1)-C(2)-C(6) 5.1(1) C(2)-C(6)-N(1)-C(7) -160.66(1) N(1)-C(5)-C(10)-C(11) -20.97(1) C(11)-N(2)-C(14)-C(15) -179.47(1) C( 16)-C( 18)-C(19)-C( 17) -111.3(1) N(2)-C(14)-C(15)-N(3) -83.65(1) C(6)-C(2)-C(3)-O(3) 173.48(1) Table 2. Hydrogen Bond Geometry (Å, °) of Compound I
D-H…A d(D-H) d (H…A) d (D-A) ZD-H…A N(3)…O(8)i 0.91 1.97 2.843(2) 160.3 N(3)-H(3B)…O(4)ii 0.91 2.26 3.024(2) 141.7 N(3)—H(3B)…O(6)iii 0.91 2.40 2.996(2) 122.9 N(3)-H(3B)-Cl(1)iv 0.91 2.90 3.442(2) 119.7 N(3)-H(3C)…O(7)v 0.91 1.90 2.745(2) 154.5 O(1)-H(1)-O(3) 0.84 1.76 2.538(2) 153.4 O(5)-H(5)…O(6)v1 0.84 1.67 2.508(2) 172.2 O(8)-H(8B)-O(7)v 0.82(2) 1.98(2) 2.793(2) 171(3) O(8)-H(8B)-O(6)v 0.82(2) 2.63(2) 3.239(2) 132(3) O(8)-H(8A)-O(4)11 0.80(2) 2.56(2) 3.249(2) 145(3) Symmetry codes: (i): –x+2, y+1/2, –z; (ii): –x+1, y–1/2, –z+1; (iii): x, y, z–1; (iv): –x+2, y–1/2, –z; (v): –x+2, y–1/2, –z+1; (vi): –x+1, y+1/2, –z+2 Table 3. C-H···π Interactions for Compound I (Å, °)
H-Cg X-H …Cg X-Cg C(18)-H(18B)…Cg(4)#1 2.83 150.0 3.719(2) Symmetry codes: #1: 2–x, –1/2+y, 1–z. Ring Cg(4)#1: N(1)→C(5)→C(4)→C(3)→C(2)→C(6)→ -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 5
- 文章访问数: 1681
- HTML全文浏览量: 144


 下载:
下载:


 下载:
下载:

