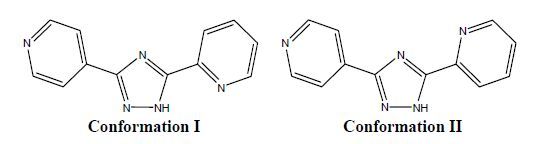
 Figure 1.
Two typical conformations of 2, 4′-Hbpt
Figure 1.
Two typical conformations of 2, 4′-Hbpt

Synthesis, Crystal Structure, Fluorescent Property and DFT Calculations of a New Zn(II) Complex Based on 3-(2-Pyridyl)-5-(4-pyridyl)-1H-1,2,4-triazole①
English
Synthesis, Crystal Structure, Fluorescent Property and DFT Calculations of a New Zn(II) Complex Based on 3-(2-Pyridyl)-5-(4-pyridyl)-1H-1,2,4-triazole①
-
1 INTRODUCTION
Currently, extensive experimental and theoretical efforts have been devoted to crystal engineering of coordination compounds from predesigned bridging ligands and metal ions, because such hybrid materials display a variety of regulated architectures and thus many potential functions[1-4]. The synthesis of coordination compounds by the judicious choice of organic spacers and metal centers can be an efficient method to obtain new types of luminescent materials, especially for d10 systems of metal centers[5]. While, choosing appropriate ligand and analyzing its most stable structure are meaningful to build regulated architectures. As a family of popular organic ligands, triazole and its derivatives have been extensively developed in metallosupramolecular assemblies[6]. The 3, 5-bis-pyridyl-1H-1, 2, 4- triazole (Hbpt) serves as a N, N′-donor ligand and acts as a bridging ligand, thus mediating the exchange coupling[7-12]. Furthermore, the prototropy and conjugation between the 1H-1, 2, 4-triazole and pyridyl groups alter the electron density in different sections of the molecules, thus making the ligand more flexible[13].
As a result, we have chosen 3-(2-pyridyl)-5-(4- pyridyl)-1H-1, 2, 4-triazole (2, 4′-Hbpt) as an organic ligand, which can be regarded as an excellent building block for the construction of new coordination compounds. Due to the flexible nature of the backbone, 2, 4′-Hbpt may exhibit two kinds of conformations (I and II) in different complexes to meet the requirements of coordination geometry (Scheme 1). In this work, a new Zn (II) coordination compound Zn (2, 4′-bpt)2(H2O) with conformation I of 2, 4′-Hbpt will be described. The compound has been structurally established by single-crystal X-ray diffraction analysis, and its thermal stability and fluorescence also have been presented. What’s more, theoretical calculation based on density functional theory (DFT) for 2, 4′-Hbpt is also employed to illuminate the stability and chemical reactivity of the ligand within different conformations, which corresponds with the experimental results of 2, 4′-Hbpt in the title complex.
2 EXPERIMENTAL
2.1 Materials and measurements
2, 4′-Hbpt was synthesized according to the Ref[14]. 2, 4′-Hbpt was further proved by IR and elemental analysis. Anal. Calcd. for C12H9N5 (%): C, 64.56; H, 4.06; N, 31.37. Found (%): C, 64.88; H, 4.56; N, 32.88. m.p: 260~261 ℃; IR (cm-1, KBr): 3408(w), 3088(m), 1622(s), 1566(s), 1473(s), 1436(m), 1315(m), 1130(s), 1045(m), 993(m), 842(s), 804(m), 746(m), 684(w), 578(w).
All commercially available chemicals were of reagent grade and used as received without further purification. Solvents were purified according to the standard methods prior to use. 1H NMR spectra were taken with a Varian 400 spectrometer using tetramethylsilane (TMS) as an internal standard. Elemental analyses (C, H and N) were performed on a Vario EL III analyzer. Infrared spectra were obtained from KBr pellets on a BEQ VZNDX 550 FTIR instrument within the 400~4000 cm-1 region. Thermogravimetric analysis was carried out on a TA Instruments NETZSCH STA 449 C simultaneous TGA at a heating rate of 10 ℃·min-1 under hydrostatic air. The luminescent spectra for the powdered solid samples were recorded at room temperature on an F-7000 FL Spectrophotometer.
2.2 Synthesis of complex 1
A mixture containing ZnSO4·7H2O (17.3 mg, 0.06 mmol), 2, 4′-Hbpt (9.0 mg, 0.03 mmol) and water (6 mL) was sealed in a 10 mL Teflon-lined stainlesssteel vessel, heated at 140 ℃ for 4 days, and then cooled to room temperature at a rate of 5 ℃·h-1. Colorless block crystals suitable for X-ray diffraction were obtained in 40% yield (based on Zn). Anal. Calcd. for C24H18ZnN10O (%): C, 54.61; H, 3.44; N, 26.54. Found (%): C, 54.78; H, 3.52; N, 26.95. IR (cm-1, KBr): 3390(w), 3080(w), 1602(s), 1573(m), 1525(m), 1465(s), 1413(m), 1263(m), 1165(m), 1031(s), 991(m), 835(s), 802(m), 750(m), 694(m), 617(w), 553(w).
2.3 X-ray crystallography
All diffraction data of complex 1 were collected on a Bruker/Siemens Smart Apex II CCD diffractometer with graphite-monochromated MoKα radiation (λ = 0.071073 nm) at 293(2) K. Cell parameters were retrieved using SMART software and refined using SAINTPLUS for all observed reflections. Data reduction and correction for Lp and decay were performed using the SAINTPLUS software. Absorption corrections were applied using SADABS. All structures were solved by direct methods using the SHELXS program of the SHELXTL-97 package and refined with SHELXL. For 2, a total of 6418 reflections were collected in the range of 2.85≤θ≤25.02°, of which 1966 were independent (Rint = 0.0893). The final R = 0.0581 and wR = 0.0898 for observed reflection with I > 2σ(I), R = 0.0911 and wR = 0.1065 for all data with (Δρ) max = 0.472 and (Δρ) min = -0.369 e·Å-3. Hydrogen bonding geometry for the title complex is collected in Table 1.
D-H…A d (D-H) d (H…A) d (D…A) ∠(DHA) O (1)-H (1)…N (3) ii 0.78(4) 1.98(4) 2.716(4) 159(5) Symmetry transformations used to generate the equivalent atoms: x, -y+1, z-1/2 Table 1. Hydrogen Bond Lengths (Å ) and Bond Angles (°) for Complex 13 RESULTS AND DISCUSSION
3.1 Structural description
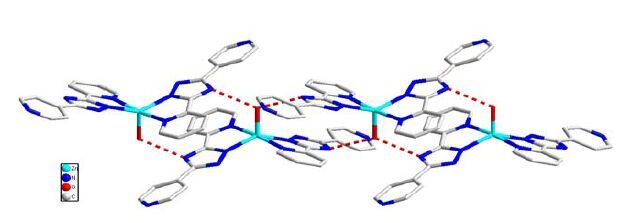
Single-crystal X-ray diffraction indicates that complex 1 is a 0D motif. The asymmetric unit consists of one Zn (II) ion, two 2, 4′-bpt- and one coordinated water molecule. The five-coordinated Zn (II) ion is surrounded by four nitrogen donors in the equatorial positions from two chelating pyridyl-triazolyl segments of different 2, 4′-Hbpt, as well as one oxygen atom in the axial position from water molecule to form a distort rectangular pyramid geometry (Fig. 1). 2, 4′-Hbpt adopts conformation I and the dihedral angles between the two pyridine planes and triazole are 13.62° and 4.18°, respectively. In addition, there is an intermolecular hydrogen bond between O (1) and N (3) (Table 2). The coordination array alternately connected by hydrogen bonds forms a corrugated 1D pattern along the [001] axis (Fig. 2).
N(2)-N(4) 1.356 1.362 N(2)-C(16) 1.337 1.344 N(4)-C(17) 1.354 1.344 N(3)-C(16) 1.366 1.352 N(3)-C(17) 1.326 1.334 N(1)-C(23) 1.341 1.338 N(1)-C(21) 1.339 1.331 N(6)-C(15) 1.346 1.339 N(6)-C(13) 1.336 1.341 N(4)-N(2)-C(16) 102.25 103.14 N(2)-N(4)-C(17) 110.87 107.46 N(2)-C(16)-N(3) 114.29 115.07 N(3)-C(17)-N(4) 110.29 112.89 C(16)-N(3)-C(17) 102.31 101.44 N(3)-C(16)-C(18) 123.24 123.38 N(3)-C(17)-C(15) 128.18 128.23 C(13)-N(6)-C(15) 117.46 118.16 C(21)-N(1)-C(23) 116.64 115.5 Table 2. Experimental and Theoretical Pa rameters for the Ligand (Conformation I)3.2 Conformational analysis
The conformation a molecule normally adopts has a deep influence on its physical and chemical properties[15]. While among the calculating methods, DFT is a favorite one due to its great accuracy in reproducing the experimental values of molecular geometry, vibrational frequencies, atomic charges, dipole moment, thermodynamical properties, etc[16-18]. So, the optimized geometrical parameters and vibrational frequencies for all structures of the conformational isomers from 2, 4′-Hbpt were calculated using DFT. The hybrid functional B3LYP with the 6-31G (d) basis set was employed without any symmetry restriction. All computations are carried out with the GAUSSIAN 09 quantum chemistry program-package[19].
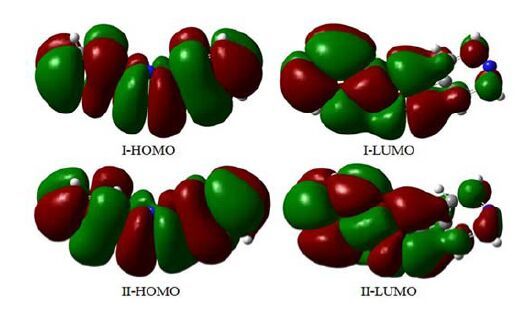
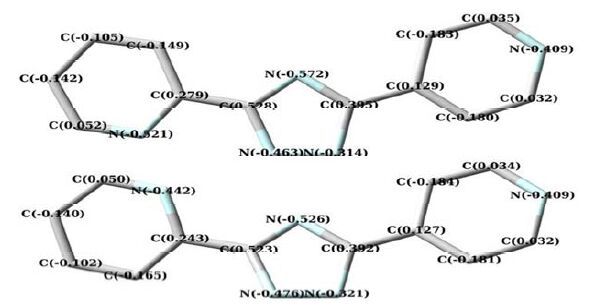
In this case, 6-31G (d) gives satisfying results, which differs from the observed parameters only by an average of 0.01 nm (Table 2), emphasizing a good choice of the level of theory[20], so the following calculations and discussions are based on the optimized structure. The energies are -736.457618109 and -736.443960479 a.u. for conformations I and II, respectively. With the point of electron energy, conformation I is more stable than II. In addition, frontier molecular orbital has also been calculated. According to the frontier molecular orbital theory, the frontier orbitals and neighboring molecular orbitals play important roles in the stability of various conformations. The LUMO is an electron acceptor and represents the ability to obtain an electron (or electrons) whereas the HOMO represents the ability to donate an electron since it is the outermost molecular orbital with electrons[21]. The DFT calculation indicates that I (and II) has 58 occupied molecular orbitals, as plotted in Fig. 3 (HOMO, LUMO). The value of the energy separation between the HOMO and LUMO was also calculated. The gap energy between the frontier molecular orbital energies is 0.3008 and 0.3001 a.u. for conformations I and II, respectively. The absolute value of the gap energy shows that conformation I can keep thermodynamic stability in the experimental conditions and not convert to other products[22]. And it also reveals conformation I has higher chemical reactivity in coordination interaction than II. What’s more, parts of atomic charges for conformations I and II are presented in Fig. 4. The charges of the N1, N4 atoms of the 2, 4′-Hbpt (conformation I) are -0.4633 and -0.5206, respectively, indicating that N1 and N4 are prone to coordination with metal ions.
The conformation a molecule normally adopts also has a deep influence on its coordination modes. Compared with conformation I, there are few relevant reports for conformation II. The literature survey also reveals that the nitrogen atom in 2-pyridine and triazole rings of conformation I is prone to chelation with metal ions[23-27], which is in agreement with the calculation results.
3.3 Thermal stability
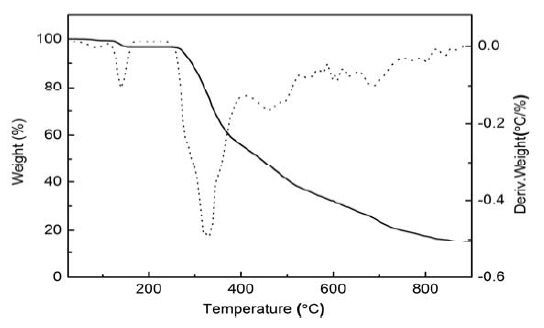
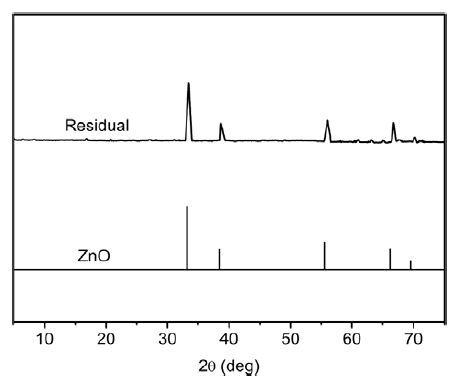
Thermogravimetric experiment is conducted to study the thermal stability of the title complex, which is an important parameter for metal-organic framework materials. As shown in Fig. 5, the TGA curve of the title complex suggests two steps of weight loss. The first weight loss of 3.26% in the range of 80~150 ℃ corresponds to the complete loss of coordinated water molecule (calcd: 3.41%). The main framework remains intact until it is heated to ca. 240 ℃, and then releases all the ligands completely from 240 to 885 ℃, giving ZnO as the final decomposition product with residue percent of 15.36% (calcd: 15.42%). The residual sample was characterized by X-ray powder diffraction (XRPD) at room temperature (Fig. 6). It is seen that all diffraction peaks agree well with the standard diffraction data for ZnO (JCPDS card file No. 65-2880).
3.4 Fluorescent properties
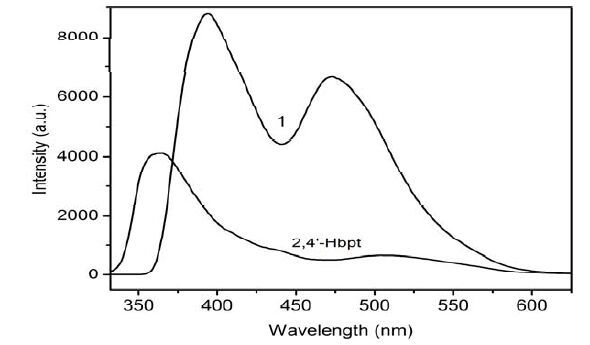
It is well known that metal-organic coordination compounds with d10 metal configuration may have roles as good candidates for potential photoactive materials because their luminescence behaviors are closely associated with the central metal ions and the ligands coordinated with them[28-30]. Taking into account the excellent luminescent properties of Zn (II) coordination compounds, the photoluminescent experiments for complex 1, as well as the free ligand, have been investigated in the solid state at room temperature (Fig. 7). For complex 1, two intense emission peaks occur at 394 and 473 nm (λex = 320 nm), respectively. To further analyze the nature of these emission bands, the photoluminescence property of the ligand 2, 4′-Hbpt has also been explored. The free 2, 4′-Hbpt molecule exhibits an intense and a weak emission peak at 365 and 503 nm (λex = 320 nm), respectively. Thus, we can presume that the emission peaks at 394 nm for complex 1 should originate from the intraligand π-π* transitions of 2, 4′-Hbpt. Furthermore, the added peak above 400 nm for complex 1 may be attributed to the ligand-to-metal charge transfer (LMCT) bands[31].
4 CONCLUSIONS
In summary, a new complex Zn (2, 4′-bpt)2(H2O) is prepared under hydrothermal reactions. In the solid state, complex 1 showed strong photoluminescent properties. It indicates that complex 1 can be used as a potential optical material. Density functional theory is successfully applied to investigate the most optimized conformation, stability, molecular frontier orbital, and charge distribution of different conformations of the ligand, which corresponds with the experimental conformation of 2, 4′-Hbpt in the complex. Accordingly, our present findings with predicting the optimized conformation of the ligand will offer a possibility of controlling the formation of desired network structures.
-
-
[1]
Jeffrey R. L., Omar M. Y.. The pervasive chemistry of metal-organic frameworks[J]. Chem. Soc. Rev., 2009, 38: 1213-1214. doi: 10.1039/b903811f
-
[2]
Du M., Li C. P., Liu C. S., Fang S. M.. Design and construction of coordination polymers with mixed-ligand synthetic strategy[J]. Coord. Chem. Rev., 2013, 257: 1282-1305. doi: 10.1016/j.ccr.2012.10.002
-
[3]
Alexander M. S., Dongwoo K., Abdelqader S., Chad A. M.. Infinite coordination polymer nano- and microparticle structures[J]. Chem. Soc. Rev., 2009, 38: 1218-1227. doi: 10.1039/b807085g
-
[4]
Li J. R., Sculley J., Zhou H. C.. Metal-organic frameworks for separations[J]. Chem. Rev., 2012, 112: 869-932. doi: 10.1021/cr200190s
-
[5]
Ali M., Masoomi M. Y.. Structures and properties of mercury(II) coordination polymers[J]. Coord. Chem. Rev., 2009, 253: 1882-1905. doi: 10.1016/j.ccr.2009.02.018
-
[6]
Zhang H., Wang X. M., Zhang K. C., Teo B. K.. Molecular and crystal engineering of a new class of inorganic cadmium-thiocyanate polymers with host-guest complexes as organic spacers, controllers, and templates[J]. Coord. Chem. Rev., 1999, 183: 157-195. doi: 10.1016/S0010-8545(98)00270-7
-
[7]
Li T., Meng Q. F., Li B., Jin X. D., Gao H., Chen X. Y., Song W. M., Liu W. Y.. Assembly of a new fluorescent Cd(II) coordination compound with a versatile multidentate N-donor building block 3-(2-pyridyl)-5-(3′-pyridyl)-1,2,4-triazole[J]. Chin. J. Struct. Chem., 2015, 34: 1525-1532.
-
[8]
Li B., Wei Q., Yang Q., Chen S. P., Gao S. L.. Synthesis, structure, and thermophysical properties of an energetic complex Co(3-(2-pyridyl)-5-(3′-pyridyl)-1H-1,2,4-triazole)3·H2O. J. Chem[J]. Eng. Data, 2011, 56: 3043-3046. doi: 10.1021/je2000364
-
[9]
Li B., Chen S. P., Xie G., Yang Q., Gao S L.. Coligand benzenedicarboxylate modulated zinc(II)-3,5-bis(3-pyridyl)-1H-1,2,4-triazole frameworks: synthesis, structure and luminescent property[J]. Chin. J. Struct. Chem., 2012, 23: 417-423. doi: 10.1007/s11224-011-9886-9
-
[10]
Li B., Shen D., Chen X. Y., Li T., Ren J. L., Hu Q. L., Liu W. Y.. A new 1-D energetic complex [Cu(2,3′-bpt)2·H2O]n: synthesis, structure and catalytic thermal decomposition for ammonium perchlorate[J]. J. Coord. Chem., 2014, 67: 2028-2038. doi: 10.1080/00958972.2014.934230
-
[11]
Han J., Li T., Li B.. A new nickel(II) coordination compound constructed by pyridyl-triazole and oxybis (benzoic acid): synthesis, crystal structure and the effect on the thermal decomposition of ammonium perchlorate[J]. Chin. J. Struct. Chem., 2015, 34: 253-259.
-
[12]
Wang J. M., Li B., Sun L., Li T., Zhou H L., Ren J L., Hu Q. L.. Synthesis, structure, thermostability and DFT calculations of a cobalt(II) coordination compound comstructed by oxybis (benzoic acid) and pyridyl-triazole[J]. Chin. J. Inorg. Chem., 2014, 30: 683-688.
-
[13]
Li B., Chen S. P., Yang Q., Gao S. L.. R-phenyldicarboxyl (R = H, NO2 and COOH) modular effect on Ni(II) coordination polymers incorporated with a versatile connector 1H-3-(3-pyridyl)-5-(4-pyridyl)-1,2,4-triazole[J]. Polyhedron, 2011, 30: 1213-1218. doi: 10.1016/j.poly.2011.01.038
-
[14]
Browne E. 124-Triazol-3-ylpyridines. Aust. J[J]. Chem., 1975, 28: 2543-2546.
-
[15]
Saravanan R. R., Seshadri S., Gunasekaran S., Mendoza-Mero?o R., Garcia-Grandad S.. Conformational analysis, X-ray crystallographic, FT-IR, FT-Raman, DFT, MEP and molecular docking studies on 1-(1-(3-methoxyphenyl) ethylidene) thiosemicarbazide[J]. Spectrochem. Acta Part A, 2015, 139: 321-328. doi: 10.1016/j.saa.2014.12.026
-
[16]
Krishnakumar V., Prabavathi N., Muthunatesan S.. Structure and vibrational frequencies of 1-naphthaldehyde based on density functional theory calculations[J]. Spectrochim. Acta A, 2008, 69: 528-533. doi: 10.1016/j.saa.2007.04.031
-
[17]
Karabacak M., Karagoz D., Kurt M.. Experimental (FT-IR and FT-Raman spectra) and theoretical (abinitio HF, DFT) study of 2-chloro-5-methylaniline[J]. J. Mol. Struct., 2008, 892: 25-31. doi: 10.1016/j.molstruc.2008.04.054
-
[18]
Shahab S., Kumar R., Darroudi M., Borzehandani M. Y.. Molecular structure and spectroscopic investigation of sodium(E)-2-hydroxy-5-((4-sulfonatophenyl)diazenyl)benzoate: a DFT study[J]. J. Mol. Struct., 2015, 1083: 198-203. doi: 10.1016/j.molstruc.2014.11.064
-
[19]
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A. Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, ?.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J.; Wallingford, C. T. Gaussian, Inc. Gaussian 09 2009.
-
[20]
Guzei I. A., Crozier K. R., Nelson K. J., Pinkert J. C., Schoenfeldt N. J., Shepardson K. E., McGaff R. W.. Synthesis and theoretical analysis of the structures and bonding in five new neutral square planar bis[2,4-di(aryl)-1,3,5-triazapentadienato] nickel(II) complexes[J]. Inorg. Chim. Acta, 2006, 359: 1169-1176. doi: 10.1016/j.ica.2005.09.022
-
[21]
Arivazhagan M., Subhasini V. P., Austine A.. Vibrational spectroscopic, first-order hyperpolarizability and HOMO, LUMO studies of 4-chloro-2-(trifluoromethyl) aniline based on DFT calculations[J]. Acta Part A, 2012, 86: 205-213. doi: 10.1016/j.saa.2011.10.026
-
[22]
Zhang J. P., Lin Y. Y., Huang X. C., Chen X. M.. Copper(I) 1,2,4-triazolates and related complexes: studies of the solvothermal ligand reactions, network topologies, and photoluminescence properties[J]. J. Am. Chem. Soc., 2005, 127: 5495-5506. doi: 10.1021/ja042222t
-
[23]
Lin J. B., Lin J. B., Xue W., Wang B. Y., Tao J., Zhang W. X., Zhang J. P., Chen X. M.. Chemical/physical pressure tunable spin-transition temperature and hysteresis in a two-step spin crossover porous coordination framework[J]. Inorg. Chem., 2012, 51: 9423-9430. doi: 10.1021/ic301237p
-
[24]
Lin J. B., Zhang J. P., Chen X. M.. Nonclassical active site for enhanced gas sorption in porous coordination polymer[J]. J. Am. Chem. Soc., 2011, 132: 6654-6656.
-
[25]
Pan J., Liu C. P., Jiang F. L., Wu M. Y., Chen L., Qian J. J., Su K. Z., Wan X. Y., Hong M. C.. Diverse architectures and luminescence properties of two novel copper(I) coordination polymers assembled from 2,6-bis[3-(pyrid-4-yl)-1,2,4-triazolyl]pyridine ligands[J]. CrystEngCommun., 2015, 17: 1541-1548. doi: 10.1039/C4CE02351J
-
[26]
Bao X., Liu J. L., Leng J. D., Lin Z. J., Tong M. L., Nihei M., Oshio H.. Spin crossover versus low-spin behaviour exhibited in 2D and 3D supramolecular isomers of [FeII(2,4-bpt)2]·guest[J]. Chem. Eur. J., 2010, 16: 7973-7978. doi: 10.1002/chem.201001179
-
[27]
F u, A. Y., Jiang , Y. L., Wa ng, Y. Y., G ao, X. N., Ya ng, G. P., H ou, L , S hi, Q. Z.. DMF/H2O volume ratio controls the syntheses and transformations of a series of cobalt complexes constructed using a rigid angular multitopic ligand[J]. Inorg. Chem., 2010, 49: 5495-5502. doi: 10.1021/ic902548f
-
[28]
Cooke M. W., Hanan G. S.. Luminescent polynuclear assemblies[J]. Chem. Soc. Rev., 2007, 36: 1466-1476. doi: 10.1039/b609200b
-
[29]
Du L. Y., Wang H., Liu G., Xie D., Guo F. S., Hou L., Wang Y. Y.. Structural diversity of five new bitriazole-based complexes: luminescence, sorption, and magnetic properties[J]. Dalton Trans., 2015, 44: 1110-1119. doi: 10.1039/C4DT03129F
-
[30]
Du Li. Y, Shi W. J, Hou L, Wang Y. Y, Shi Q. Z, Zhu Z. H[J]. Solvent or temperature induced diverse coordination polymers of silver(I) sulfate and bipyrazole systems: syntheses, crystal structures, luminescence, and sorption properties. Inorg. Chem., 2013, 52: 14018-14027.
-
[31]
Du M., Jiang X. J., Zhao X. J.. Molecular tectonics of mixed-Ligand metal-organic frameworks: positional isomeric effect, metal-directed assembly, and structural diversification[J]. Inorg. Chem., 2007, 46: 3984-3995. doi: 10.1021/ic062098+
-
[1]
-
Table 1. Hydrogen Bond Lengths (Å ) and Bond Angles (°) for Complex 1
D-H…A d (D-H) d (H…A) d (D…A) ∠(DHA) O (1)-H (1)…N (3) ii 0.78(4) 1.98(4) 2.716(4) 159(5) Symmetry transformations used to generate the equivalent atoms: x, -y+1, z-1/2 Table 2. Experimental and Theoretical Pa rameters for the Ligand (Conformation I)
N(2)-N(4) 1.356 1.362 N(2)-C(16) 1.337 1.344 N(4)-C(17) 1.354 1.344 N(3)-C(16) 1.366 1.352 N(3)-C(17) 1.326 1.334 N(1)-C(23) 1.341 1.338 N(1)-C(21) 1.339 1.331 N(6)-C(15) 1.346 1.339 N(6)-C(13) 1.336 1.341 N(4)-N(2)-C(16) 102.25 103.14 N(2)-N(4)-C(17) 110.87 107.46 N(2)-C(16)-N(3) 114.29 115.07 N(3)-C(17)-N(4) 110.29 112.89 C(16)-N(3)-C(17) 102.31 101.44 N(3)-C(16)-C(18) 123.24 123.38 N(3)-C(17)-C(15) 128.18 128.23 C(13)-N(6)-C(15) 117.46 118.16 C(21)-N(1)-C(23) 116.64 115.5 -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 9
- 文章访问数: 1689
- HTML全文浏览量: 173



 下载:
下载:







 下载:
下载:

