 Figure Figure1.
(a) A rhombic ring constructed with pztcH2 2- anion itself by the H-bonds; (b) Protonated 4, 4΄-bipyH2 2+ cation acting as a bridge to connect the two layers, and the purple dashed line shows the π-π interaction
Figure Figure1.
(a) A rhombic ring constructed with pztcH2 2- anion itself by the H-bonds; (b) Protonated 4, 4΄-bipyH2 2+ cation acting as a bridge to connect the two layers, and the purple dashed line shows the π-π interaction

pH Dependent Supramolecules Based on Co-crystallization of Pyrazine-2,3,5,6-tetracarboxylic Acid with 4,4'-Bipyridine through Intermolecular Hydrogen Bonds①
English
pH Dependent Supramolecules Based on Co-crystallization of Pyrazine-2,3,5,6-tetracarboxylic Acid with 4,4'-Bipyridine through Intermolecular Hydrogen Bonds①
-
Key words:
- pyrazine-2,3,5,6-tetracarboxylic acid
- / 4,4'-bipyridine
- / hydrogen bond
-
1 INTRODUCTION
Supramolecular chemistry is a rapidly expanding area of solid-state chemical research due to its potential applications in the field of molecular biology, materials science and pharmaceutical science[1]. The ultimate goal of supramolecular chemistry is to control the molecular assembly and orientation within a solid-state structure, which will further lead to predictable and desired structural aggregates[2]. An important approach to control supramolecular crystal structure is the consideration of robust and reliable intermolecular interactions, in which H-bonds often play a dominant role due to their selectivity and directionality to control the design of various molecular assemblies[3]. For example, H-bonds have been extensively used in the field of organic crystal engineering to assemble organic molecular building blocks into well-defined crystalline materials[4]. From a supramolecular perspective, the binary components with high symmetry mole- cules represent an illustration of how one might exploits the modular approach to design new supra- molecular architectures, especially in the solid state[5].
It is known that pyrazine-2, 3, 5, 6-tetracarboxylic acid (pztcH4) has been chosen as a building unit for the supermolecules because it is a molecule with predictable and interesting supramolecular properties (interpenetration, polymorphism and inclusion) as a consequence of its molecular symmetry and complementary H-bonding capabilities[6]. This carboxylic acid can form homo- or heterodimers with a variety of complementary functional groups[7]. On the other hand, 4, 4΄-bipy as another building unit is able to generate supramolecular organizations in crystal engineering[8]. Until now, many transition metal coordinated polymers based on pztcH4, 4, 4΄-bipy or both have been intensively reported[9]. The self-assembly process between the two pure organic materials 4, 4΄-bipy and pztcH4 leading to robust H-bonded supramolecular aggregates has not reported yet.
In this work, we focused on the preparation and structural characterization of two binary organic ionic salts with formulas [(4, 4΄-bipyH2)(pztcH2)] (1) and [(4, 4΄-bipyH2)(pztcH3)(Cl)]·4H2O (2). Compounds 1 and 2 were obtained based on the same starting materials pztcH4 and 4, 4΄-bipy, but in two different pH conditions adjusted with HCl acid. H-bonds of various types are found in 1 and 2 which allow our detailed study of H-bonding networks in these charged organic molecules.
2 EXPERIMENTAL
2.1 Reagents and instruments
The pztcH4 ligand was synthesized according to the literatures[10]. All the other reagents were of analytical grade and used without further purification. Solvents were freshly distilled by using appropriate drying agents prior to use. 1H NMR and 13C NMR spectra were recorded on an AVANCE DRX 400 (Bruker) in DMSO-d6. Infrared data were recorded on an FT-IR spectrometer (Scimitar 2000, Varian) by using KBr pellets in the range of 4000~ 400 cm−1. Elemental analyses for C, H, and N were performed on a Heraeus CHN-O-Rapid.
2.2 Synthesis of [(4, 4΄-bipyH2)(pztcH2)] (1)
To a solution of pztcH4 (25.6 mg, 0.1 mmol) in distilled water (20 mL) was slowly added a solution of 4, 4΄-bipy (15.6 mg, 0.1 mmol) in methanol (5 mL). The mixed solution was stirred overnight to produce lots of white precipitates, which were completely dissolved after the pH value of solution was adjusted to 3~4 with concentrated HCl. Silver gray crystals were obtained after standing the solution for a few weeks. Yield: 41.4 mg, 56%. IR (KBr, cm−1): 3426, 3093, 3057, 2972, 1704, 1628, 1597, 1489, 1385, 1260, l167, 1093, 1047, 880. 1H NMR (400 MHz, DMSO-d6): 8.73(m, 1H), 7.86(m, 1H). 13C NMR (400 MHz, DMSO-d6): 165.34, 150.76, 145.59, 145.16, 122.02. Anal. Calcd. for C9H6N2O4 (Fw = 206.15): C, 52.43; H, 2.93; N, 13.59%. Found (%): C, 52.67/52.83; H, 2.96/2.96; N, 13.67/13.81.
2.3 Synthesis of [(4, 4΄-bipyH2)(pztcH3)(Cl)]·4H2O (2)
To a solution of pztcH4 (25.6 mg, 0.1 mmol) in distilled water (2 mL) was dropwisely added a solution of 4, 4΄-bipy (15.6 mg, 0.1 mmol) in methanol (2 mL) to give white flocky precipitates. After being stirred for 1 h, the pH value of the reacting solution was adjusted to 1~2 with concentrated HCl to completely dissolve the white precipitates. The solution was filtered and the filtrate was standing quietly for ten days to give light yellow crystals. Yield: 23.8 mg, 57.8%. IR (KBr, cm−1): 3431, 3108, 3073, 1711, 1651, 1479, 1381, 1253, 1166, 1139, 1000, 811. 1H NMR (400 MHz, DMSO-d6): 8.94(m, 1H), 8.22(m, 1H). 13C NMR (400 MHz, DMSO-d6): 165.26, 147.80, 145.52, 123.56. Anal. Calcd. for C18H21ClN4O12 (Fw = 520.84): C, 41.51, H, 4.06, N, 10.76%. Found (%): C, 41.66/41.74; H, 4.16/4.08; N, 10.82/10.90.
2.4 Structure determination
X-ray diffraction data for the single crystals of compounds 1 and 2 were collected on a Bruker Smart CCD diffractometer with graphite-monochromated Mo-Kα radiation (0.71073 Å). The structures were solved by direct methods using the SHELXS program of SHELXTL package and refined with SHELXL[11]. In crystal 1, 2880 reflections were independent with Rint = 0.0440, collected in the 2.29≤θ≤24.99º range, of which 1424 were considered to be observed (I > 2σ(I)) and used in the succeeding refinement. While in 2, 7681 reflections were independent with Rint = 0.0318, collected in the region of 2.41≤θ≤25.0º, of which 1973 were considered to be observed (I > 2σ(I)) and used in the succeeding refinement. The final refinements of both compounds were performed by full-matrix leastsquares methods on F2 with anisotropic thermal parameters for all non-H atoms. H atoms attached to C were placed geometrically and allowed to ride during the subsequent refinement with an isotropic displacement parameter fixed at 1.2 times Ueq of the parent atoms. While H atoms bound to O or N were located in difference Fourier maps. The final refinement including hydrogen atoms converged to R = 0.0360, wR = 0.0973, S = 1.025, (Δρ)max = 0.261 and (Δρ)min = −0.203 e/Å3 in 1; and R = 0.0343, wR = 0.0978, S = 1.021, (Δρ)max = 0.167 and (Δρ)min = −0.231 e/Å3 in 2.
3 RESULTS AND DISCUSSION
3.1 Synthesis and characterization
In the preparation of 1 and 2, the direct combination of acid in water and base in methanol with molar ratio 1:1 led to the formation of a large amount of white precipitation, which was completely dissolved after the pH of the reacting solution was adjusted to 3~4 in the former and 1~ 2 in the latter with HCl. Corresponding crystals are obtained after standing the clear solution quietly for weeks. Compounds 1~2 have been characterized by IR spectrum, NMR and elemental analysis. In the IR spectra, the strong absorptions at 1704 (1) and 1711 (2) cm−1 are characteristic for the asymmetric stretching vibration of C=O group in carboxylate acid, which are some blue-shifted compared with that in ligand pztcH4 (1747 cm−1). The strong bands at about 1651 and 1596 cm−1 belong to the vibration of aromatic rings of pyridine and benzene. EA and NMR technologies also proved the syntheses of compounds 1 and 2.
3.2 Structural analysis
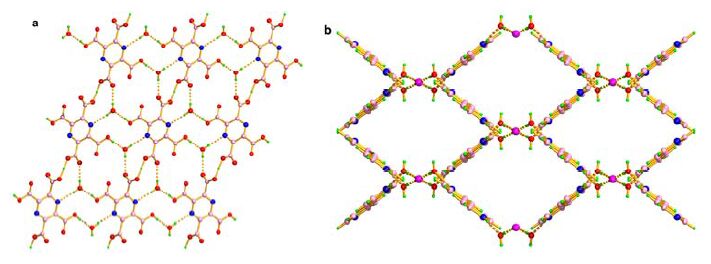
Compound 1 crystallizes in the triclinic system with space group P 1 , and the asymmetric unit contains a half protonated 4, 4΄-bipyH2 2+ cation and a half pztcH2 2− anion. Every pztcH2 2− anion is linked with its neighbors through two pairs of strong H-bonds (Oacid−H···Oacid = 2.4428(17) Å, Oacid− H···Oacid = 2.4611(18) Å) to construct a rhombic ring which is further linked by the same type of H-bonds, creating an extended honeycomb topology, as shown in Fig. 1a. Further analysis of the crystal packing indicates that these 2-D layers take on a parallel stacking mode, with the distance between two adjacent layers to be ca. 5.775 Å. In the channel of the 2-D layers, the protonated 4, 4΄-bipyH2 2+ cations work as bridges to connect the neighboring layers with the two H-bonds (Nbipy−H···Oacid = 2.598(2) Å) to form a 3-D structure (Fig. 1b). At last, in the channel, the 4, 4΄-bipy ring is nearly parallel to its neighbor with the centroid-to-centroid distance being 3.7653(14) Å, indicating π-π stacking interactions between the pyridinyl rings (Fig. 1b).
 Figure Figure1.
(a) A rhombic ring constructed with pztcH2 2- anion itself by the H-bonds; (b) Protonated 4, 4΄-bipyH2 2+ cation acting as a bridge to connect the two layers, and the purple dashed line shows the π-π interaction
Figure Figure1.
(a) A rhombic ring constructed with pztcH2 2- anion itself by the H-bonds; (b) Protonated 4, 4΄-bipyH2 2+ cation acting as a bridge to connect the two layers, and the purple dashed line shows the π-π interaction
Compound 2 crystallizes in the monoclinic system with C2/c space group, and the asymmetric unit contains a half protonated 4, 4΄-bipyH2 2+ cation, a half pztcH3 − anion, a half Cl− anion, and two solvated water molecules. Similar to compound 1, each pztcH3− unit builds up chains with two H-bonds (Oacid−H···Oacid = 2.4599(16) Å) in the vertical direction as shown in Fig. 2a, which are further linked with the adjacent chains by one water molecule O6 using Ow−H···Npyim, Ow−H···Oacid, and Oacid−H···Ow H-bonds to afford a 3-D lattice network (Fig. 2b). On the other hand, the 4, 4΄-bipyH22+ cation, Cl− anion and the other water molecule O5 are linked into another chain with H-bonds, and these chains are linked with each other with two different directions through H-bonds to afford a grid. The above formed two grids are crisscrossed to give the finial 3-D framework connected with H-bonds. To be noticed, each water molecule held in the cavities works as a μ3-connected node to link the pztcH3− anion, 4, 4΄- bipyH2 2+ cation, and Cl− anion using H-bonds to form the finial 3-D network.
3.3 Hydrogen bond
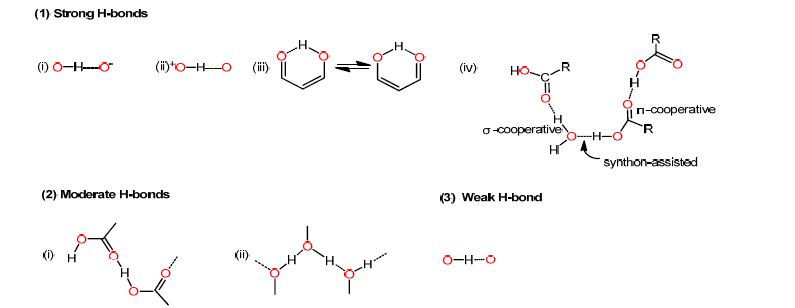
The intermolecular H-bond plays an important role in the co-crystals. Based on J. A. K. Howard's work, the H-bonds have been categorized as shown in Scheme 1[12]. (1) Very strong H-bonds included four main types: (i) the negative charge-assisted H-bonds; (ii) positive charge-assisted H-bonds; (iii) the resonance-assisted H-bonds; (iv) the synthonassisted H-bond through synergistic π- and σ-cooperativity. (2) Moderate H-bonds consist of polarization- assisted, either (i) π-cooperative, or (ii) σ-cooperative H-bonds. (3) Weak, isolated H-bonds are neither charge nor π-or σ-cooperative. For understanding the H-bond in compounds 1 and 2, we got deep inside into their bond lengths and bond angles, as shown in Table 1.
D-H…A d(D-H) (A) d(H-A) (A) d(D…A) (A) ZDHA (°) Compound 1 O(1)-H(5)-O(1)a 1.22 1.22 2.4428(17) 180 O(3)-H(6)-O(3)b 1.23 1.23 2.4611(18) 180 N(1)-H(7)-O(2)a 0.98(3) 1.62(3) 2.598(2) 173(3) Compound 2 O(4)-H(4A)…O(4)c 1.2300(12) 1.2300(12) 2.4599(16) 180(3) O(2)-H(2A)…O(6) 0.88(3) 1.69(3) 2.551(2) 168(3) N(1)-H(1A)…O(5) 0.90(3) 1.83(3) 2.659(2) 154(2) O(5)-H(5A)-Cl(1) 0.81(3) 2.26(3) 3.0723(18) 178(4) O(5)-H(5B)-O(3) 0.77(3) 2.04(3) 2.801(2) 169(3) O(6)-H(6B)…N(2)d 0.80(3) 2.16(3) 2.955(2) 177(2) O(6)-H(6A)…O(3)e 0.83(2) 2.02(2) 2.8310(19) 166(2) Symmetric codes: a = 1 – x, 1 – y, –z; b = –x, –y, 1 – z; c = 1 – x, y, 1/2 – z; d = 3/2 – x, 1/2 – y, 1 – z; e = 1/2 + x, 1/2 – y, 1/2 + z In compound 1, the pztcH22− acid itself assembles to form a 2-D layer with two robust H-bonds (O(1)−H(5)···O(1B), O(3)−H(6)···O(3A)), as listed in Table 1. The bond lengths are 2.4428(17) and 2.4611(18) Å and the bond angles are close to 180°, which explained that both are ascribed to the negative charge-assisted H-bonds. The decrease in the O···O distance is accompanied by a lengthening of the O−H bond and a shortening of the H···O bond until a symmetrical H-bond is reached. In 1, the other type of H-bond N(1)−H(7)···O(2) is between the deprotonated 4, 4΄-bipyH22+ and pztcH22− acid in the 2-D layer. The bond length and bond angle are 2.598(2) Å and 173(3)°, revealing it belongs to the π-assisted H-bond (Table 1). Thus, two strong and one moderate H-bonds make a contribution to the formation of the co-crystals of 1.
While in compound 2, pztcH3− acid also self-assembles to form a 1-D chain through strong negative charge-assisted H-bonds (O(4)−H(4A)···O(4) = 2.4599(16) Å, ∠OHO = 180(3)°, Table 1). Between chains, water molecule O(6) has three types of H-bonds to connect with pztc. One very short H-bond (O(2)−H(2A)···O(6) = 2.551(2) Å, ∠OHO =168(3)° is ascribed to the synthon-assisted H-bond in which the COOH donor is activated by π-cooperative H-bonding, and the water-acceptor ability is enhanced by polarization assistance (σ-cooperative). The other two H-bonds (O(6)− H(6A)···O(3) = 2.8310(19) Å; O(6)−H(6B)···N(2) = 2.955(2) Å) are assigned to the σ-cooperative H-bonds. Similarly, in water O5, there are also three H-bonds (O(5)−H(5B)···O(3) = 2.801(2) Å, O(5)− H(5A)···Cl(1) = 3.0723(18) Å, N(1)−H(1A)···O(5) = 2.659(2) Å), with the former two to be σ-cooperative and the latter one to be synthon-assisted.
4 CONCLUSION
In this research, we described the synthesis and characterization of two co-crystals based on pztcH4 and 4, 4΄-bipy components. It is revealed the pH value and various H-bonds fulfill the diversiform motives of compounds 1 and 2. Especially, the negative charge-assisted and synthon-assisted H-bonds have significant role in the construction of stable supramolecular adducts. It is suggested the very short intermolecular H-bonds are essentially three-center-four-electron covalent bonds with the length shortened and the covalent character increased.
-
-
[1]
(a) Desiraju, G. R. Crystal engineering: from molecule to crystal. Journal of the American Chemical Society 2013, 135, 9952–9967; (b) Bosch, E.; Radford, R.; Barnes, C. L. Donor-acceptor interactions in crystal engineering. Organic Letters 2001, 3, 881–883.
-
[2]
(a) Biradha, K.; Su, C. Y.; Vittal, J. J. Recent developments in crystal engineering. Crystal Growth & Design 2011, 11, 875–886; (b) Babu, N. J.; Nangia, A. Multiple Z' in carboxylic acid-pyridine trimer synthon and Kagome lattice in the structure of 5-methylpyrazine-2,3-dicarboxylic acid. Crystal Growth & Design 2006, 6, 1995–1999.
-
[3]
(a) Tian, D.; Pang, Y.; Guo, S.; Zhu, X.; Zhang, H. Syntheses, crystal structures, and magnetic properties of two new manganese(II) complexes based on biphenyl-2,5,2΄,5΄-tetracarboxylic acid. Journal of Coordination Chemistry 2011, 64, 1006–1015; (b) Thuery, P.; Masci, B. Lanthanide-organic assemblies with pyrazinetetracarboxylic and benzophenone-3,3΄,4,4΄-tetracarboxylic acids. Crystengcomm 2010, 12, 2982–2988; (c) Masci, B.; Thuery, P. Pyrazinetetracarboxylic acid as an assembler ligand in uranyl-organic frameworks. Crystal Growth & Design 2008, 8, 1689–1696; (d) Du, M.; Zhang, Z. H.; Zhao, X. J. Cocrystallization of trimesic acid and pyromellitic acid with bent dipyridines. Crystal Growth & Design 2005, 5, 1247–1254.
-
[4]
(a) Zhang, H.; Guo, C.; Wang, X.; Xu, J.; He, X.; Liu, Y.; Liu, X.; Huang, H.; Sun, J. Five energetic cocrystals of BTF by intermolecular hydrogen bond and pi-stacking interactions. Crystal Growth & Design 2013, 13, 679–687; (b) Babu, N. J.; Nangia, A. Water-mediated multicenter synthon and aromatic C-H -> N isostructurality. Crystal Growth & Design 2006, 6, 1753–1756; (c) Fabelo, O.; Canadillas-Delgado, L.; Delgado, F. S.; Lorenzo-Luis, P.; Laz, M. M.; Julve, M.; Ruiz-Perez, C. Hydrogen bond-directed frameworks based on 1,2,4,5-benzene-tetracarboxylate. Crystal Growth & Design 2005, 5, 1163–1167.
-
[5]
Seaton C. C., Parkin A.. Making benzamide cocrystals with benzoic acids: the influence of chemical structure[J]. Crystal Growth & Design, 2011, 11: 1502.
-
[6]
(a) Yang, A. H.; Quan, Y. P.; Gao, H. L.; Fang, S. R.; Zhang, Y. P.; Zhao, L. H.; Cui, J. Z.; Wang, J. H.; Shi, W.; Cheng, P. ds-Block metal ions catalyzed decarboxylation of pyrazine-2,3,5,6-tetracarboxylic acid and the complexes obtained from hydrothermal reactions and novel water clusters. Crystengcomm 2009, 11, 2719–2727; (b) Yang, A. H.; Gao, H. L.; Cui, J. Z.; Zhao, B. Syntheses, structures, and photoluminescence of lanthanide coordination polymers with pyridine-2,3,5,6-tetracarboxylic acid. Crystengcomm 2011, 13, 1870–1876; (c) Zhao, L. H.; Quan, Y. P.; Yang, A. H.; Cui, J. Z.; Gao, H. L.; Lu, F. L.; Shi, W.; Cheng, P. Syntheses and crystal structures of two new nickel(II) complexes with pyrazine-2,3,5,6-tetracarboxylate. Crystengcomm. 2009, 11, 1427–1432; (d) Quan, Y. P.; Zhao, L. H.; Yang, A. H.; Cui, J. Z.; Gao, H. L.; Lu, F. L.; Shi, W.; Cheng, P. Novel lanthanide coordination polymers based on bis-tridentate chelator pyrazine-2,3,5,6-tetracarboxylate with nano-channels and water clusters. Crystengcomm. 2009, 11, 1679–1685.
-
[7]
(a) Masci, B.; Pasquale, S.; Thuery, P. Alkali metal ion complexes with pyrazinetetracarboxylate: two- and three-dimensional frameworks. Crystal Growth & Design 2010, 10, 2004–2010; (b) Ghosh, S. K.; Bharadwaj, P. K. Infinite chains of quasi-planar hexameric water clusters stabilized in a metal-organic framework built from Co-II and pyrazine-2,3,5,6-tetracarboxylic acid. European Journal of Inorganic Chemistry 2005, 2005, 4880–4885.
-
[8]
(a) Zheng, S. R.; Pan, M.; Wu, K.; Chen, L.; Jiang, J. J.; Wang, D. W.; Shi, J. Y.; Su, C. Y. Assembly of binuclear, tetranuclear, and multinuclear complexes from pincer-like mononuclear metallotectons: structural diversity dependent on precursors. Crystal Growth & Design 2015, 15, 625–634; (b) Zhang, H. M.; Yang, J.; Liu, Y. Y.; Kang, D. W.; Ma, J. F. A family of coordination polymers assembled with a flexible hexacarboxylate ligand and auxiliary N-donor ligands: syntheses, structures, and physical properties. Crystengcomm. 2015, 17, 3181–3196.
-
[9]
(a) Gao, H. L.; Zhang, Q. Q.; Cheung, C. W.; Yi, Y. L.; Li, F. F.; Qu, J.; Jiang, S. X.; Shi, X. Y.; Cui, J. Z. Syntheses, structures and properties of silver(I) complexes constructed from nitrogenous aromatic heterocyclic carboxylic acids and N-donor ligands. Inorganic Chemistry Communications 2014, 46, 194–197; (b) Zhang, F.; Yan, P.; Zou, X.; Zhang, J.; Hou, G.; Li, G. Novel 3D alkali-lanthanide heterometal-organic frameworks with pyrazine-2,3,5,6-tetracarboxylic acid: synthesis, structure, and magnetism. Crystal Growth & Design 2014, 14, 2014–2021; (c) Shi, X. Y.; Yang, A. H.; Qu, J.; Chen, W. T.; Gao, H. L.; Cui, J. Z. Syntheses, structures, and fluorescent properties of three ds-block metal complexes with pyrazine-2,3,5,6-tetracarboxylic acid and 4,4΄-bipyridine. Chinese Journal of Inorganic Chemistry 2014, 30, 2174–2180; (d) Li, F. F.; Zhang, Q. Q.; Zhao, Y. Y.; Jiang, S. X.; Shi, X. Y.; Cui, J. Z.; Gao, H. L. Syntheses, structures, and properties of six new coordination polymers constructed from N-heterocyclic multicarboxylic acids. Rsc Advances 2014, 4, 10424–10433; (e) Zhai, B.; Yi, Y. L.; Han, N. N.; Zhang, X. F. Synthesis, crystal structure and magnetic research of 1D chain manganese coordination polymer based on pyrazine-2,3,5,6-tetracarboxylic acid. Chinese Journal of Inorganic Chemistry 2012, 28, 2535–2539; (f) Fu, F.; Li, D. S.; Wu, Y. P.; Gao, X. M.; Du, M.; Tang, L.; Zhang, X. N.; Meng, C. X. A versatile V-shaped tetracarboxylate building block for constructing mixed-ligand Co(II) and Mn(II) complexes incorporating various N-donor co-ligands. Crystengcomm. 2010, 12, 1227–1237; (g) Fang, M. J.; Li, M. X.; He, X.; Shao, M.; Pang, W.; Zhu, S. R. Synthesis, structure and thermal stability of ternary metal complexes based on polycarboxylate and N-heterocyclic ligands. Journal of Molecular Structure 2009, 921, 137–143.
-
[10]
(a) Ai-Hong, Y.; Hong-Ling, G.; Jian-Zhong, C. A hexagonal C 3-symmetric (H2O) 7 guest fits well into a C 3-symmetric terbium complex cavity. Inorganic Chemistry Communications 2010, 13, 1309–1313; (b) Hong-Ling, G.; Yan-Ping, Z.; Ai-Hong, Y.; Su-Rong, F.; Jian-Zhong, C. 1D slide-fastener-like coordination polymers of Mn(II) derived from pyrazine-2,3,5,6-tetracarboxylic acid. Journal of Molecular Structure 2009, 918, 97–100.
-
[11]
(a) Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallographica Section C-Structural Chemistry 2015, 71, 3–8; (b) Sheldrick, G. M. A short history of SHELX. Acta Crystallographica Section A 2008, 64, 112–122.
-
[12]
(a) Vishweshwar, P.; Babu, N. J.; Nangia, A.; Mason, S. A.; Puschmann, H.; Mondal, R.; Howard, J. A. K. Variable temperature neutron diffraction analysis of a very short O–H center dot center dot center dot O hydrogen bond in 2,3,5,6-pyrazinetetracarboxylic acid dihydrate: synthon-assisted short O-acid-H center dot center dot center dot O-water hydrogen bonds in a multicenter array. Journal of Physical Chemistry A 2004, 108, 9406–9416; (b) Ghosh, S. K.; Bharadwaj, P. K. Structure of a discrete hexadecameric water cluster in a metal-organic framework structure. Inorganic Chemistry 2004, 43, 6887–6889; (c) Fourmigue, M.; Batail, P. Activation of hydrogen- and halogen-bonding interactions in tetrathiafulvalene-based crystalline molecular conductors. Chemical Reviews 2004, 104, 5379–5418.
-
[1]
-
Table 1. H-bonds in Compounds 1 and 2
D-H…A d(D-H) (A) d(H-A) (A) d(D…A) (A) ZDHA (°) Compound 1 O(1)-H(5)-O(1)a 1.22 1.22 2.4428(17) 180 O(3)-H(6)-O(3)b 1.23 1.23 2.4611(18) 180 N(1)-H(7)-O(2)a 0.98(3) 1.62(3) 2.598(2) 173(3) Compound 2 O(4)-H(4A)…O(4)c 1.2300(12) 1.2300(12) 2.4599(16) 180(3) O(2)-H(2A)…O(6) 0.88(3) 1.69(3) 2.551(2) 168(3) N(1)-H(1A)…O(5) 0.90(3) 1.83(3) 2.659(2) 154(2) O(5)-H(5A)-Cl(1) 0.81(3) 2.26(3) 3.0723(18) 178(4) O(5)-H(5B)-O(3) 0.77(3) 2.04(3) 2.801(2) 169(3) O(6)-H(6B)…N(2)d 0.80(3) 2.16(3) 2.955(2) 177(2) O(6)-H(6A)…O(3)e 0.83(2) 2.02(2) 2.8310(19) 166(2) Symmetric codes: a = 1 – x, 1 – y, –z; b = –x, –y, 1 – z; c = 1 – x, y, 1/2 – z; d = 3/2 – x, 1/2 – y, 1 – z; e = 1/2 + x, 1/2 – y, 1/2 + z -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 16
- 文章访问数: 2010
- HTML全文浏览量: 218


 下载:
下载:


 下载:
下载:

