
Synthesis, Crystal Structure and Fluorescent Property of a Zn(II) Complex with 1,3-Bis(imidazol-1-yl)benzene and 1,3-Benzenedicarboxylate①
English
Synthesis, Crystal Structure and Fluorescent Property of a Zn(II) Complex with 1,3-Bis(imidazol-1-yl)benzene and 1,3-Benzenedicarboxylate①
-
Key words:
- biimidazole
- / zinc metal complex
- / crystal structure
- / fluorescent property
-
1 INTRODUCTION
Over the last two decades, the chemists have devoted themselves to the development of new crystalline materials with a variety of functions, properties, and potential applications, such as fluorescence[1-5], magnetism[6-12], catalysis[13], sensing material[14], gas sorption[15, 16], etc. The factors influencing the construction of MOFs are complicated. In particular, the organic ligands have significant influences on the desirable MOFs because of conformational preferences, steric hindrance effects, flexibility, and so on. Thus, the prospect of controlling the structures and properties of MOFs by the selection of suitable organic ligands has attracted more interest in the researches on metalorganic frameworks[17-27].
The ligand BIB[1, 3-bis(imidazol-1-yl)benzene] is a V-shaped flexible ligand. The flexible nature of spacers allows the ligands to bend and rotate when they coordinate to metal centers, which often causes structural diversity[28-32]. The bdc(1, 3-benzenedicarboxylate) is a bent linker, having been demonstrated excellent coordinating ability, giving various novel complexes with the structure aberrancy, and its complexes may have novel networks[33-35]. In order to test the ability of this ligand to give new architectures, we use BIB ligand and aromatic carboxylic acid bdc as a co-ligand to coordinate with the metal Zn(II) cation, obtaining a novel complex with intriguing structure, [Zn(BIB)(bdc)]n (1).
2 EXPERIMENTAL
2.1 Materials and general procedures
All chemicals and solvents were commercially purchased and used without further purification. The BIB ligand was synthesized according to the reported method[36]. Elemental analysis was carried out on a Perkin-Elmer 240C elemental analyzer. Infrared (IR) spectra were recorded on a Bruker Vector 22 FTIR spectrophotometer by using KBr discs. Powder X-ray diffraction (PXRD) patterns were obtained on a Philips X-pert X-ray diffractometer. Thermogravimetric analyses (TGA) were performed on a Perkin Elmer Pyris II thermal analyzer in nitrogen at a heating rate of 20 ℃⋅min-1. The luminescence spectra for the powdered solid samples were measured on a SHIMAZU VF-320Xray fluorescence spectrophotometer at room temperature upon excitation at 331 nm (gap at 1.5 mm).
2.2 Synthesis of the complex
A mixture of Zn(NO3)2·6H2O (29.7 mg, 0.1 mmol), BIB (21.0 mg, 0.1 mmol), and bdc (16.6 mg, 0.1 mmol) was dissolved in 9 mL of DMF/H2O (2:1, v/v). The final mixture was sealed in a 15 mL Parr Teflon-lined stainless-steel vessel and heated at 85 ℃ for 3 days, and then the reaction system was cooled to room temperature. Colorless rectangular block crystals of complex 1 were obtained, washed with water, and dried under ambient conditions (Yields: 54% for complex 1 based on Zn). Elemental analysis calcd. for (C20H14N4O4Zn) (%): C, 54.58; H, 3.18; N, 12.74. Found (%): C, 54.55; H, 3.16; N, 12.77. IR (KBr, cm-1): 1612(vs), 1515(s), 1353(vs), 1263(m), 1059(s), 899(w), 818(m), 745(vs), 649(m).
2.3 Crystal structure determination
A single crystal with dimensions of 0.30mm × 0.28mm × 0.26mm was selected and determined by a Bruker Smart Apex Ⅱ CCD diffractometer with a graphite-monochromatic MoKα radiation (λ = 0.71073 Å) by using a φ-ω scan mode at 293(2) K. Out of the total 32536 reflections collected in the range of 2.45≤θ≤27.56º, 8460 were independent with Rint = 0.0501, of which 7072 were considered to be observed (I > 2σ(I)). The structure of complex 1 was solved by direct methods and refined on F2 by full-matrix least-squares technique[37]. All nonhydrogen atoms were refined anisotropically and the hydrogen atoms were added theoretically. The selected bond lengths and bond angles are given in Table 1. The final refinement gives R = 0.0541, wR = 0.0935 (w = 1/[σ2(Fo 2) + (aP)2 + bP], where P = (Fo 2 + 2Fc 2)/3), Goodness-of-fit is 1.027, (Δρ)max = 0.711 and (Δρ)min = -0.481 e/Å3.
Bond Dist Bond Dist Zn(1)-N(1) 2.017(2) Zn(2)-N(5) 2.029(2) Zn(1)-N(4)#4 2.013(2) Zn(2)-N(8)#4 2.039(2) Zn(1)-O(1) 1.9377(18) Zn(2)-O(5) 1.9411(19) Zn(1)-O(4)#3 1.974(2) Zn(2)-O(8)#3 1.9611(18) Angle (°) Angle (°) O(1)-Zn(1)-O(4)#3 118.99(8) O(5)-Zn(2)-O(8)#3 122.74(8) O(1)-Zn(1)-N(4)#4 104.82(9) O(5)-Zn(2)-N(5) 116.66(9) O(4)#3-Zn(1)-N(4)#4 110.15(9) O(8)#3-Zn(2)-N(5) 95.86(9) O(1)-Zn(1)-N(1) 120.78(9) O(5)-Zn(2)-N(8)#4 100.47(9) O(4)#3-Zn(1)-N(1) 95.16(9) O(8)#3-Zn(2)-N(8)#4 111.61(9) N(4)#4-Zn(1)-N(1) 106.26(9) N(5)-Zn(2)-N(8)#4 109.64(9) Symmetry codes: #3 = x, –1+y, z. #4 = 1+x, y, z 3 RESULTS AND DISCUSSION
3.1 Structure description of the crystal
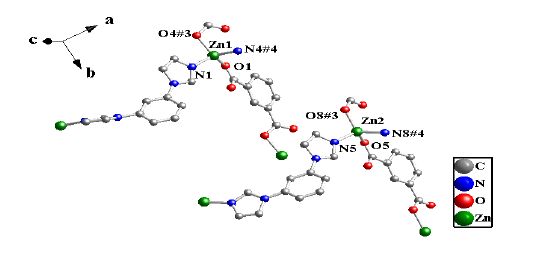
Single-crystal X-ray analysis reveals that complex 1 is a 2D coordination polymer, monoclinic crystal system of P2/c. Complex 1 contains two crystallographically independent Zn(II) cations (Zn(1) and Zn(2)) that are four-coordinated with two O atoms (O(1), O(4#3) and O(5), O(8#3), respectively) of the two bdc molecules and two N atoms of the two BIB molecules (N(1), N(4#4) and N(5), N(8#4), respectively). The Zn-O lengths fall in the range of 1.9377(18) ~ 1.974(2) Å, the Zn-N lengths are 2.013(2) ~ 2.039(2) Å, which are all in good agreement with those typically observed[38], as shown in Fig. 1. The Co(Ⅱ) complex (2) contains two crystallographically independent Co(II) cations (Co(1 )and Co(2))[29], but the Co(Ⅱ) cations adopt two different coordination modes. Co(1) and Co(2) adopt six- and five-coordination modes, respectively. The Co(II) cations (Co(1) and Co(2)) are coordinated with O atoms from the bdc molecules together with two N atoms from two BIB molecules. The Co(Ⅱ) complex (2) is a 2D coordination polymer in triclinic space group P1.
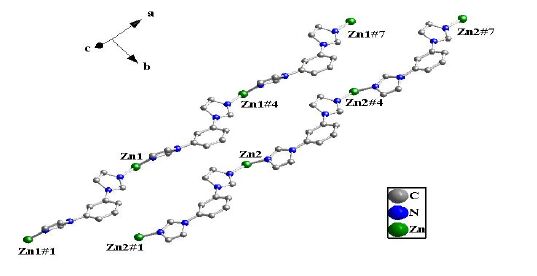
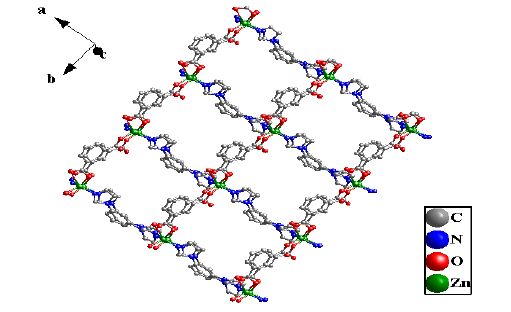
The crystal unit contains two [Zn(BIB)] units, which are chemically identical while not related by crystallographic symmetry. The [Zn(BIB)] units are connected to each other, which leads to a neutral double 1D polymeric chain structure due to the different rotation degree for the BIB ligand. There exist two different double 1D polymeric chains in complex 1 (Fig. 2). Two different 1D chains are connected by the bdc ligands to form a 2D coordination framework (Fig. 3). It is interesting that two BIB and bdc ligands link four Zn(II) cations to form an approximately rectangular [Zn4(BIB)2(bdc)2] 36-membered ring, with the diagonal of rectangle to be 15.041(1) Å (Fig. 3).
3.2 Thermal stability and powder X-ray diffraction (PXRD)
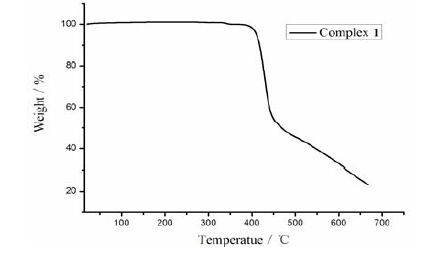
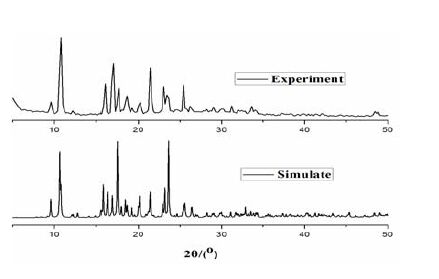
Thermogravimetric analysis of complex 1 (TGA, Supporting Information, Fig. 4) shows that no weight loss is observed until it reached 396 ℃, at which the decomposition of complex 1 occurred. To confirm whether the crystal structure is truly representative of the bulk material, powder X-ray diffraction analysis (PXRD) experiment was carried out for complex 1. The measured peak positions closely match the simulated ones (Fig. 5). They show that the bulk synthesized materials and the measured single crystals are the same.
3.3 Fluorescence property of the title complex
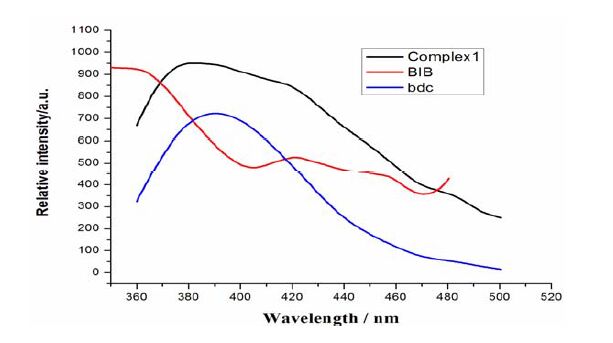
The complexes with d10 metal centers have been investigated for fluorescent properties with potential applications in photochemistry, chemical sensors, and light-emitting diodes[39]. The luminescent spectra of complex 1 and the ligands were examined in the solid state at room temperature (Fig. 6). Emissions of the ligands were observed with wavelengths at 351 nm in BIB (λex = 331 nm) and 388 nm (λex = 331 nm) in bdc, which both could be attributed to the π*-π transitions. Complex 1 showed an intense broad photoluminescence with maximum emission at 391 nm upon excitation at 331 nm, which is close to the emission at 388 nm of the bdc ligand under the same conditions. Therefore, the emission observed in complex 1 is attributed to the π-π* intraligand photoluminescence due to its resemblance to that of the bdc ligand. And the enhancement and slight red-shift of complex 1 compared to that of the bdc ligand probably result from the fact that the coordination of Zn(II) ions increases the ligand conformational rigidity and thus reduces the loss of energy through a radiationless pathway[40].
4 CONCLUSION
In summary, a novel zinc metal complex 1 has been successfully synthesized under solvothermal conditions. Complex 1 is a two-dimensional doublelayer structure. The alliance of BIB and bdc is good for diversity of the getatable compound structure. Additionally, complex 1 displays better fluorescence and thermal stability.
-
-
[1]
Zhao X. H., Zhao Y. Y., Zhang J., Pan J. G., Li X.. Syntheses, crystal structures and fluorescent spectra of two Zn(II) complexes with sulfonamide pyrimidine[J]. Chin. J. Inorg. Chem., 2014, 30: 633-639.
-
[2]
Yu J. H., Yao R., Yuan L. M., Xu B., Qu B. T., Liu W. L.. Cd(II) coordination polymers constructed from flexible disulfide ligand: solvothermal syntheses, structures and luminescent properties[J]. Inorg. Chim. Acta, 2011, 376: 222-229. doi: 10.1016/j.ica.2011.06.020
-
[3]
Feng R., Jiang F. L., Chen L., Yan C. F., Wu M. Y., Hong M. C.. A luminescent homochiral 3D Cd(II) framework with a threefold interpenetrating uniform net 86. Chem[J]. Commun., 2009, : 5296-5298.
-
[4]
Hu J. S., Shang Y. J., Yao X. Q., Qin L., Li Y. Z., Guo Z. J., Zheng H. G., Xue Z. L.. Syntheses, structures, and photochemical properties of six new metal-organic frameworks based on aromatic dicarboxylate acids and V-shaped imidazole ligands[J]. Cryst. Growth Des., 2010, 10: 4135-4142. doi: 10.1021/cg1008208
-
[5]
Hu J. S., Shang Y. J., Yao X. Q., Qin L., Li Y. Z., Guo Z. J., Zheng H. G., Xue Z. L.. Syntheses, structures, and photoluminescence of five new metal-organic frameworks based on flexible tetrapyridines and aromatic polycarboxylate acids[J]. Cryst. Growth Des., 2010, 10: 2676-2684. doi: 10.1021/cg1001557
-
[6]
Yang Q. X., Huang L. F., Zhang M. D.. An unprecedented homochiral metal-organic framework based on achiral nanosized pyridine and V-shaped polycarboxylate acid ligand[J]. Cryst. Growth Des., 2013, 13: 440-445. doi: 10.1021/cg301600x
-
[7]
Xu J., Pan Z. R., Wang T. W., Li Y. Z., Guo Z. J., Batten S. R., Zheng H. G.. Syntheses structures, photoluminescence and magnetic properties of five compounds with1,3,5-benzenetricarboxylate acid and imidazole ligands[J]. CrystEngComm., 2010, 12: 612-619. doi: 10.1039/B916222D
-
[8]
Xu J., Yao X. Q., Huang L. F., Li Y. Z., Zheng H. G.. Syntheses, structures, photoluminescence and magnetic properties of four new metal-organic frameworks based on imidazole ligands and aromatic polycarboxylate acids[J]. CrystEngComm., 2011, 13: 857-865. doi: 10.1039/C0CE00219D
-
[9]
Simon M., Humphrey R. A., Mole M. R., Paul T. W.. Hydrothermal synthesis and magnetic properties of novel Mn(II) and Zn(II) materials with thiolato-carboxylate donor ligand frameworks[J]. Dalton Trans., 2004, : 1670-1678.
-
[10]
Hu J. S., Huang L. F., Yao X. Q., Qin L., Li Y. Z., Guo Z. J., Zheng H. G., Xue Z. L.. Six new metal-organic frameworks based on polycarboxylate acids and V-shaped imidazole-based synthon: syntheses, crystal structures, and properties[J]. Inorg. Chem., 2011, 50: 2404-2414. doi: 10.1021/ic102207n
-
[11]
Qin L., Hu J. S., Huang L. F., Li Y. Z., Guo Z. J., Zheng H. G.. Syntheses, characterizations, and properties of six metal-organic complexes based on flexible ligand 5-(4-pyridyl)-methoxyl isophthalic acid[J]. Cryst. Growth Des., 2010, 10: 4176-4183. doi: 10.1021/cg100870v
-
[12]
Qin L., Hu J. S., Li Y. Z., Zheng H. G.. Effect of carboxylate coligands with different rigidity on supramolecular architectures based on one rigid didentate linear ligand[J]. Cryst. Growth Des., 2012, 12: 403-413. doi: 10.1021/cg2012638
-
[13]
Ma L., Abney C., Lin W.. Enantioselective catalysis with homochiral metal-organic frameworks[J]. Chem. Soc. Rev., 2009, 38: 1248-1256. doi: 10.1039/b807083k
-
[14]
Lu Z. Z., Zhang R., Li Y. Z., Guo Z. J., Zheng H. G.. Solvatochromic behavior of a nanotubular metal-organic framework for sensing small molecules[J]. J. Am. Chem. Soc., 2011, 133: 4172-4174. doi: 10.1021/ja109437d
-
[15]
Jiang L., Meng X. R., Xiang H., Ju P., Zhong D. C., Lu T. B.. Variations of structures and gas sorption properties of three coordination polymers induced by fluorine atom positions in azamacrocyclic ligands[J]. Inorg. Chem., 2012, 51: 1874-1880. doi: 10.1021/ic202168r
-
[16]
Qin L., Hu J. S., Li Y. Z., Zhang M. D., Guo Z. J., Zheng H. G.. Structure-property relationship of homochiral and achiral supramolecular isomers obtained by one-pot synthesis[J]. Chem. Commun., 2012, 48: 10757-10759. doi: 10.1039/c2cc36003a
-
[17]
Feng X., Wang L. Y., Shi X. G., Zhao J., Sun Q.. A dicyanamido-bridged zinc(II) complex based on the bis(4-pyridyl)ethane with a three-dimensional framework[J]. Chin.J. Struct. Chem., 2010, 29: 1567-1573.
-
[18]
Chen Y. C., Wang K. B., Wang Y.. A one-dimensional transition metal complex constructed from 24-membered hinge-shaped macrocycles with salicylic acid and 1,3-bis(4-pyridyl)propane[J]. Chin. J. Struct. Chem., 2009, 28: 1613-1618.
-
[19]
Zhong D. C., Deng J. H., Luo X. Z., Liu H. J., Zhong J. L., Wang K. J., Lu T. B.. Two cadmium-cluster-based metal-organic frameworks with mixed ligands of 1,2,3-benzenetriazole (HBTA) and 1,4-benzenedicarboxylic acid (H2BDC). Cryst[J]. Growth Des., 2012, 12: 1992-1998. doi: 10.1021/cg2016963
-
[20]
Du M., Li C. P., Liu C. S., Fang S. M.. Design and construction of coordination polymers with mixed-ligand synthetic strategy[J]. Coord. Chem. Rev., 2013, 257: 1282-1305. doi: 10.1016/j.ccr.2012.10.002
-
[21]
Murugavel, R.; Baheti, K.; Anantharaman, G. Reactions of 2-mercaptobenzoic acid with divalent alkaline earth metal ions: synthesis, spectral studies, and single-crystal X-ray structures of calcium, strontium, and barium complexes of 2,2΄-dithiobis(benzoic acid). Inorg Chem. 2001, 40, 6870-6878.
-
[22]
Grant A., Broker R. P. A., Edward R. T. T.. Co-crystallization of 2,2΄-dithiodibenzoic acid with the isomeric n-pyridinealdazines, n = 2, 3 and 4:supramolecular polymers and the influence of steric factors upon aggregation patterns[J]. CrystEngComm., 2008, 10: 879-887. doi: 10.1039/b800797g
-
[23]
Huang W. H., Hou L., Liu B., Cui L., Wang Y. Y., Shi Q. Z.. Two novel interpenetrating MOFs constructed from a derivative of phenanthroline and a V-shaped flexible dicarboxylate ligand contains unique chiral structure[J]. Inorg.Chim Acta, 2012, 382: 13-18. doi: 10.1016/j.ica.2011.09.053
-
[24]
Bhubon S. R. K., Abdul M. K. M., Ozra G. M. S., Mohamed S. E. F., Karand S. M. N. K.. A novel maze-type network assembled by copper(II) and 2,2΄-dicarboxydiphenylthioether:[J]. Inorg. Chem.Commun., 2001, 4: 315-318. doi: 10.1016/S1387-7003(01)00200-3
-
[25]
Chan, M. H. E.; Crouse, K. A.; Tahir, M. I. M.; Rosli, R.; Umar-Tsafe, N.; Cowley, A. R. Synthesis and characterization of cobalt(II), nickel(II), copper(II), zinc(II) and cadmium(II) complexes of benzyl N-[1-(thiophen-2-yl)ethylidene] hydrazine carbodithioate and benzyl N-[1-(thiophen-3- yl)ethylidene] hydrazine carbodithioate and the X-ray crystal structure of bis{benzyl N-[1-(thiophen-2-yl)ethylidene] hydrazine carbodithioate}nickel(II). Polyhedron 2008, 27, 1141-1149.
-
[26]
McCann M., Coyle B., McKay S., McCormack P., Kavanagh K., Devereux M., McKee V., Kinsela P., Connor R. O., Clynes M.. Synthesis and X-ray crystal structure of[J]. Biometals, 2004, 17: 635-654. doi: 10.1007/s10534-004-1229-5
-
[27]
Thati , B , Noble , A , Creaven , B. S.. RETRACTED: in vitro anti-tumour and cyto-selective effects of coumarin-3-carboxylic acid and three of its hydroxylated derivatives, along with their silver-based complexes, using human epithelial carcinoma cell lines.[J]. Cancer Letters, 2007, 248: 321-331. doi: 10.1016/j.canlet.2006.08.009
-
[28]
Hu J. S., Yao X. Q., Zhang M. D., Qin L., Li Y. Z., Guo Z. J., Zheng H. G., Xue Z . L.. Syntheses, structures, and characteristics of four new metal-organic frameworks based on flexible tetrapyridines and aromatic polycarboxylate acids[J]. Cryst. Growth Des., 2012, 12: 3426-3435. doi: 10.1021/cg201362x
-
[29]
Zhang C. L., Wang H. Y., Qin L., Zheng H. G.. Synthesis, crystal structure and property of two Co(II) coordination polymers based on 1,3-bis(imidazol-1-yl) benzene and m-phthalic acid[J]. Inorg. Chem. Commun., 2015, 31: 303-308.
-
[30]
Zhang C. L., Qin L., Zheng H. G.. Synthesis, characterization and crystal structure of one Mn(II) complex with 4,4΄-bisimidazolylbiphenyl and 4,4΄-sulfonyldibenzoic c acid[J]. Inorg. Chem. Commun., 2013, 34: 34-36. doi: 10.1016/j.inoche.2013.05.006
-
[31]
Ji C. C., Qin L., Li Y. Z., Guo Z. J., Zheng H. G.. Effect of different imidazole ancillary ligands on supramolecular architectures of a series of Zn(II) and Cd(II) complexes with a bent dicarboxylate ligand[J]. Cryst. Growth Des., 2011, 11: 480-487. doi: 10.1021/cg101261f
-
[32]
Zhang C. L., Qin L., Xu J. G., Zheng H. G.. Synthesis, crystal structure and optical properties of Ni(II) coordination polymer constructed by 4,4΄-biphenyl imidazole and isophthalic acid ligands[J]. Inorg. Chem. Commun., 2013, 11: 2347-2350.
-
[33]
Zhang C. L., Qin L., Zheng H. G.. Synthesis, characterization and crystal structure of one Co(II) complex with 4,4΄-bisimidazolylbiphenyl and m-phthalic acid[J]. Inorg. Chem. Commun., 2013, 36: 192-194. doi: 10.1016/j.inoche.2013.09.014
-
[34]
Zhang C. L., Qin L.. Synthesis, crystal structure and properties of the 1D Ni(II) coordination polymer based on 1,3-bis( imidazol-1-yl) benzene and m-phthalic acid[J]. J. Synth. Cryst., 2013, 11: 2467-2470.
-
[35]
Zhang C. L., Wang H. Y., Qin L., Zheng H. G.. Synthesis, crystal structure and magnetic property of the 2D Mn(Ⅱ) coordination polymer based on 4,4΄-bisimidazolylbiphenyl and m-phthalic acid[J]. Inorg. Chem. Commun., 2013, 10: 2157-2161.
-
[36]
Laura S., Volodymyr B., Ronny G.. Structural diversity of cobalt(II) coordination compounds involving bent imidazole ligand: a route from 0D dimer to 3D coordination polymer[J]. Polyhedron, 2012, 44: 179-186. doi: 10.1016/j.poly.2012.06.065
-
[37]
Sheldrick, G. M. SHELXL 97, Program for the Refinement of Crystal Structure. University of Göttingen Göttingen, Germany 1997.
-
[38]
Deng Y. F., Chen M. S., Zhang C. H.. Synthesis, crystal structure and photoluminescent property of Zn(II) complex constructed from1,3-benzenedicarboxylate and1,4-di(1-imidazoly)benzene[J]. Chin. J. Inorg. Chem., 2013, 29: 2195-2199.
-
[39]
Allendorf M. D., Bauer C. A., Bhakta R. K., Houk R. J. T.. Luminescent metal-organic frameworks[J]. Chem. Soc. Rev., 2009, 38: 1330-1334. doi: 10.1039/b802352m
-
[40]
Lakowicz, J. R. Principles of fluorescence spectroscopy. Plenum Press: New York 1983.
-
[1]
-
Table 1. Selected Bond Lengths (Å) and Bond Angles (°) for Complex 1
Bond Dist Bond Dist Zn(1)-N(1) 2.017(2) Zn(2)-N(5) 2.029(2) Zn(1)-N(4)#4 2.013(2) Zn(2)-N(8)#4 2.039(2) Zn(1)-O(1) 1.9377(18) Zn(2)-O(5) 1.9411(19) Zn(1)-O(4)#3 1.974(2) Zn(2)-O(8)#3 1.9611(18) Angle (°) Angle (°) O(1)-Zn(1)-O(4)#3 118.99(8) O(5)-Zn(2)-O(8)#3 122.74(8) O(1)-Zn(1)-N(4)#4 104.82(9) O(5)-Zn(2)-N(5) 116.66(9) O(4)#3-Zn(1)-N(4)#4 110.15(9) O(8)#3-Zn(2)-N(5) 95.86(9) O(1)-Zn(1)-N(1) 120.78(9) O(5)-Zn(2)-N(8)#4 100.47(9) O(4)#3-Zn(1)-N(1) 95.16(9) O(8)#3-Zn(2)-N(8)#4 111.61(9) N(4)#4-Zn(1)-N(1) 106.26(9) N(5)-Zn(2)-N(8)#4 109.64(9) Symmetry codes: #3 = x, –1+y, z. #4 = 1+x, y, z -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 3
- 文章访问数: 1488
- HTML全文浏览量: 122


 下载:
下载:





 下载:
下载:

