Citation:
Jiao-Xue YANG, Guo-Chun LV, Yan WANG, Ze-Hua WANG, Xiao-Min SUN, Zhi-Qiang LI. Mechanism Studies on the Degradation of Hexachlorocyclopentadiene in the Atmosphere[J]. Chinese Journal of Structural Chemistry,
;2020, 39(11): 1925-1932.
doi:
10.14102/j.cnki.0254–5861.2011–2833

-
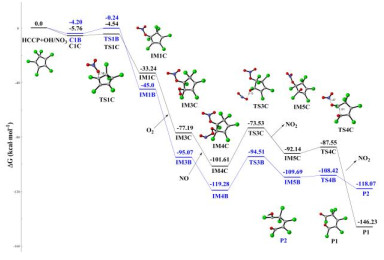
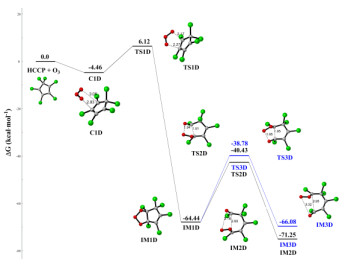
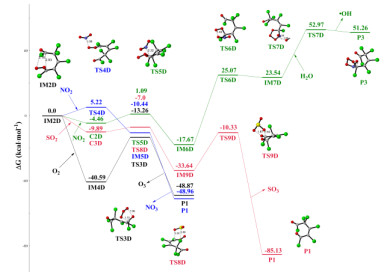
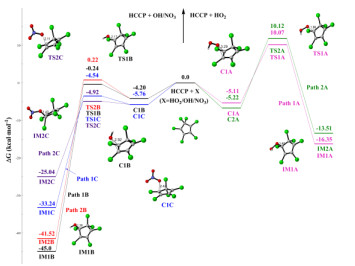
Hexachlorocyclopentadiene (HCCP) is one of the chlorinated and highly reactive pollutants, which can be released into the atmosphere and undergo chemical transformations. In this paper, the initiated reaction mechanisms of HCCP with typical atmospheric oxidants (•NO3, •HO2, •OH, and O3) were theoretically investigated. The results mean that all initiated reactions are exothermic, and the energy barriers do not exceed 16 kcal⋅mol-1. The rate constants of HCCP reaction triggered by •NO3, •HO2, •OH, and O3 are 2.49 × 10-12, 2.44 × 10-22, 2.46 × 10-13 and 1.33 × 10-20 cm3⋅molecule-1⋅s-1 at 298 K, respectively. It can be concluded that the reaction of •NO3 and •OH with HCCP more likely occurs according to the rate constants. Then the subsequent reactions of the •NO3/•OH-initiated intermediates with O2 and NO were calculated, resulting in that the cyclopentadiene is ruptured completely. And the results show that the Criegee intermediates created in the ozonization reactions of HCCP can react with O2, NO2 and SO2. This study gives more insight into the chemical transformation mechanisms of HCCP in the atmosphere.
-

-
-
[1]
McBee, E. T.; Hatton, R. E. Production of hexachlorobutadiene. Ind. Eng. Chem. 1949, 41, 809−812. doi: 10.1021/ie50472a031
-
[2]
Dharmarathne, N. K.; Mackie, J. C.; Kennedy, E. M.; Stockenhuber, M. Mechanism and rate of thermal decomposition of hexachlorocyclopentadiene and its importance in PCDD/F formation from the combustion of cyclodiene pesticides. J. Phys. Chem. A 2017, 121, 5871−5883. doi: 10.1021/acs.jpca.7b05209
-
[3]
Robitschek, P.; Bean, C. T. Flame-resistant polyesters from hexachlorocyclopentadiene. Ind. Eng. Chem. 1954, 46, 1628−1632. doi: 10.1021/ie50536a034
-
[4]
Zhang, C.; Wang, D.; Song, J.; Li, C.; Mo, Y. Bonding of Diels-Alder reactions of substituted 2-borabicyclo(1.1. 0)but-1(3)-enes: a theoretical study. Theor. Chem. Acc. 2019, 138, 106−5. doi: 10.1007/s00214-019-2491-5
-
[5]
Lu, P. Y.; Metcalf, R. L.; Hirwe, A. S.; Williams, J. W. Evaluation of environmental distribution and fate of hexachlorocyclopentadiene, chlordene, heptachlor, and heptachlor epoxide in a laboratory model ecosystem. J. Agric. Food Chem. 1975, 23, 967−973. doi: 10.1021/jf60201a016
-
[6]
Podowski, A. A.; Sclove, S. L.; Pilipowicz, A.; Khan, M. A. Q. Biotransformation and disposition of hexachlorocyclopentadiene in fish. Arch. Environ. Contam. Toxicol. 1991, 20, 488−496. doi: 10.1007/BF01065837
-
[7]
Rand, G. M.; Nees, P. O.; Calo, C. J.; Alexander, D. J.; Clark, G. C. Effects of inhalation exposure to hexachlorocyclopentadiene on rats and monkeys. J. Toxicol. Env. Health Part A 1982, 9, 743−760. doi: 10.1080/15287398209530201
-
[8]
Abdo, K. M.; Montgomery, C. A.; Kluwe, W. M.; Farnell, D. R.; Prejean, J. D. Toxicity of hexachlorocyclopentadiene: subchronic (13-week) administration by gavage to F344 rats and B6C3F1, mice. J. Appl. Toxicol. 1984, 4, 75−81. doi: 10.1002/jat.2550040204
-
[9]
Spehar, R. L.; Veith, G. D.; DeFoe, D. L.; Bergstedt, B. V. Toxicity and bioaccumulation of hexachlorocyclopentadiene, hexachloronorbornadiene and heptachloronorbornene in larval and early juvenile fathead minnows, Pimephales promelas. Bull. Environ. Contam. Toxicol. 1979, 21, 576−583. doi: 10.1007/BF01685472
-
[10]
Dharmarathne, N. K.; Mackie, J. C.; Lucas, J.; Kennedy, E. M.; Stockenhuber, M. Mechanisms of thermal decomposition of cyclodiene pesticides, identification and possible mitigation of their toxic products. Proc. Combust. Inst. 2019, 37, 1143−1150. doi: 10.1016/j.proci.2018.06.121
-
[11]
Sun, X.; Zhang, C.; Zhao, Y.; Bai, J.; Zhang, Q.; Wang, W. Atmospheric chemical reactions of 2, 3, 7, 8-tetrachlorinated dibenzofuran initiated by an OH radical: mechanism and kinetics study. Environ. Sci. Technol. 2012, 46, 8148−8155. doi: 10.1021/es301413v
-
[12]
Lin, L. F.; Lee, W. J.; Li, H. W.; Wang, M. S.; Chang-Chien, G. P. Characterization and inventory of PCDD/F emissions from coal-fired power plants and other sources in Taiwan. Chemosphere 2007, 68, 1642−1649. doi: 10.1016/j.chemosphere.2007.04.002
-
[13]
Yan, M.; Qi, Z.; Yang, J.; Li, X.; Ren, J.; Xu, Z. Effect of ammonium sulfate and urea on PCDD/F formation from active carbon and possible mechanism of inhibition. J. Environ, Sci. 2014, 26, 2277−2282. doi: 10.1016/j.jes.2014.09.012
-
[14]
Nubbe, M. E.; Adams, V. D.; Moore, W. M. The direct and sensitized photo-oxidation of hexachlorocyclopentadiene. Water Res. 1995, 29, 1287−1293. doi: 10.1016/0043-1354(94)00276-D
-
[15]
Wolfe, N. L.; Zepp, R. G.; Schlotzhauer, P.; Sink, M. Transformation pathways of hexachlorocyclopentadiene in the aquatic environment. Chemosphere 1982, 11, 91−101. doi: 10.1016/0045-6535(82)90160-6
-
[16]
Stemmler, E. A.; Hites, R. A. Methane enhanced negative ion mass spectra of hexachlorocyclopentadiene derivatives. Anal Chem. 1985, 57, 684−692. doi: 10.1021/ac00280a025
-
[17]
Shi, J.; Liu, H.; Sun, L.; Hou, H.; Xu, Y.; Wang, Z. Theoretical study on hydrophilicity and thermodynamic properties of polyfluorinated dibenzofurans. Chemosphere 2011, 84, 296−304. doi: 10.1016/j.chemosphere.2011.04.016
-
[18]
Zhou, Q.; Sun, X.; Gao, R.; Hu, J. Mechanism and kinetic properties for OH-initiated atmospheric degradation of the organophosphorus pesticide diazinon. Atmos. Environ. 2011, 45, 3141−3148. doi: 10.1016/j.atmosenv.2011.02.064
-
[19]
Gao, R.; Sun, X.; Yu, W.; Zhang, Q.; Wang, W. Mechanism and rate constants for complete series reactions of 19 fluorophenols with atomic H. J. Environ. Sci. 2014, 26, 154−159. doi: 10.1016/S1001-0742(13)60392-7
-
[20]
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery Jr., J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, N. J.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, Ö.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision B. 01, Gaussian, Inc., Wallingford CT 2009.
-
[21]
Wang, Y.; Verma, P.; Jin, X.; Truhlar, D. G.; He, X. Revised M06 density functional for main-group and transition-metal chemistry. Proc. Natl. Acad. Sci. U. S. A 2018, 115, 10257−10262. doi: 10.1073/pnas.1810421115
-
[22]
Zhao, Y.; Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215−241. doi: 10.1007/s00214-007-0310-x
-
[23]
Li, C.; Xie, H. B.; Chen, J.; Yang, X.; Zhang, Y.; Qiao, X. Predicting gaseous reaction rates of short chain chlorinated paraffins with ·OH: overcoming the difficulty in experimental determination. Environ. Sci. Technol. 2014, 48, 13808−13816. doi: 10.1021/es504339r
-
[24]
Zheng, J.; Frisch, M. J. Efficient geometry minimization and transition structure optimization using interpolated potential energy surfaces and iteratively updated hessians. J. Chem. Theory Comput. 2017, 13, 6424−6432. doi: 10.1021/acs.jctc.7b00719
-
[25]
Hratchian, H. P.; Schlegel, H. B. Using Hessian updating to increase the efficiency of a Hessian based predictor-corrector reaction path following method. J. Chem. Theory Comput. 2005, 1, 61−69. doi: 10.1021/ct0499783
-
[26]
Maeda, S.; Harabuchi, Y.; Ono, Y.; Taketsugu, T.; Morokuma, K. Intrinsic reaction coordinate: calculation, bifurcation, and automated search. Int. J. Quantum Chem. 2015, 115, 258−269. doi: 10.1002/qua.24757
-
[27]
Truhlar, D. G.; Garrett, B. C.; Klippenstein, S. J. Current status of transition-state theory. J. Phys. Chem. 1996, 100, 12771−12800. doi: 10.1021/jp953748q
-
[28]
Pechukas, P. Transition state theory. Annu. Rev. Phys. Chem. 1981, 32, 159−177. doi: 10.1146/annurev.pc.32.100181.001111
-
[29]
Berndt, T.; Böge, O. Atmospheric reaction of OH radicals with 1, 3-butadiene and 4-hydroxy-2-butenal. J. Phys. Chem. A 2007, 111, 12099−12105. doi: 10.1021/jp075349o
-
[30]
Cheng, G. J.; Zhang, X.; Chung, L. W.; Xu, L.; Wu, Y. D. Computational organic chemistry: bridging theory and experiment in establishing the mechanisms of chemical reactions. J. Am. Chem. Soc. 2015, 137, 1706−1725. doi: 10.1021/ja5112749
-
[31]
Truong, T. N. Reaction class transition state theory: hydrogen abstraction reactions by hydrogen atoms as test cases. J. Chem. Phys. 2000, 113, 4957−4964. doi: 10.1063/1.1287839
-
[32]
Canneaux, S.; Bohr, F.; Henon, E. KiSThelP: a program to predict thermodynamic properties and rate constants from quantum chemistry results. J. Comput. Chem. 2014, 35, 82−93. doi: 10.1002/jcc.23470
-
[33]
Lan, Y.; Wheeler, S. E.; Houk, K. N. Extraordinary difference in reactivity of ozone (OOO) and sulfur dioxide (OSO): a theoretical study. J. Chem. Theory Comput. 2011, 7, 2104−2111. doi: 10.1021/ct200293w
-
[34]
Wadt, W. R.; Goddard, W. A. Electronic structure of the Criegee intermediate. Ramifications for the mechanism of ozonolysis. J. Am. Chem. Soc. 1975, 97, 3004−3021. doi: 10.1021/ja00844a016
-
[35]
Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental implications of hydroxyl radicals (•OH). Chem. Rev. 2015, 115, 13051−13092. doi: 10.1021/cr500310b
-
[36]
Tsuji, M.; Kawahara, T.; Uto, K.; Hayashi, J.; Tsuji, T. Photochemical removal of NO2 in air at atmospheric pressure using side-on type 172-nm Xe2 excimer lamp. Int. J. Environ. Sci. Technol. 2019, 16, 5685−5694. doi: 10.1007/s13762-018-2131-y
-
[37]
Zelenov, V. V.; Aparina, E. V.; Kozlovskiy, V. I.; Sulimenkov, I. V.; Nosyrev, A. E. Kinetics of NO3 uptake on pyrene as a representative organic aerosols. Russ. J. Phys. Chem. B 2018, 12, 343−351. doi: 10.1134/S1990793118020136
-
[38]
Li, W.; Chen, Y.; Tong, S.; Guo, Y.; Zhang, Y.; Ge, M. Kinetic study of the gas-phase reaction of O3 with three unsaturated alcohols. J. Environ. Sci. 2018, 71, 292−299. doi: 10.1016/j.jes.2018.04.009
-
[39]
Logan, J. A. Tropospheric ozone: seasonal behavior, trends, and anthropogenic influence. Journal of Geophysical Research: Atmos 1985, 90, 10463−10482. doi: 10.1029/JD090iD06p10463
-
[40]
Lawrence, M. G.; Jöckel, P.; von Kuhlmann, R. What does the global mean OH concentration tell us? Atmos. Chem. Phys. 2001, 1, 37−49. doi: 10.5194/acp-1-37-2001
-
[41]
Holland, F.; Hofzumahaus, A.; Schäfer, J.; Kraus, A.; Pätz, H. W. Measurements of OH and HO2 radical concentrations and photolysis frequencies during BERLIOZ. J. Geophys. Res. Atmos. 2003, 108, 2−23.
-
[42]
Vrekoussis, M.; Kanakidou, M.; Mihalopoulos, N.; Crutzen, P. J.; Lelieveld, J.; Perner, D.; Berresheim, H.; Baboukas, E. Role of the NO3 radicals in oxidation processes in the eastern Mediterranean troposphere during the MINOS campaign. Atmos. Chem. Phys. 2004, 4, 169−182. doi: 10.5194/acp-4-169-2004
-
[43]
Horie, O.; Moortgat, G. K. Decomposition pathways of the excited Criegee intermediates in the ozonolysis of simple alkenes. Atmos. Environ. 1991, 25, 1881−1896. doi: 10.1016/0960-1686(91)90271-8
-
[44]
Taatjes, C. A.; Meloni, G.; Selby, T. M.; Trevitt, A. J.; Osborn, D. L.; Percival, C. J.; Shallcross, D. E. Direct observation of the gas-phase Criegee intermediate (CH2OO). J. Am. Chem. Soc. 2008, 130, 11883−11885. doi: 10.1021/ja804165q
-
[45]
Jiang, L.; Xu, Y. S.; Ding, A. Z. Reaction of stabilized Criegee intermediates from ozonolysis of limonene with sulfur dioxide: ab initio and DFT study. J. Phys. Chem. A 2010, 114, 12452−12461. doi: 10.1021/jp107783z
-
[46]
Long, B.; Bao, J. L.; Truhlar, D. G. Atmospheric chemistry of Criegee intermediates: unimolecular reactions and reactions with water. J. Am. Chem. Soc. 2016, 138, 14409−14422. doi: 10.1021/jacs.6b08655
-
[47]
Richter, A.; Burrows, J. P. Tropospheric NO2 from GOME measurements. Adv. Space Res. 2002, 29, 1673−1683. doi: 10.1016/S0273-1177(02)00100-X
-
[1]
-

-
-
[1]
Shaohua Zhang , Liyao Liu , Yingqiao Ma , Chong-an Di . Advances in theoretical calculations of organic thermoelectric materials. Chinese Chemical Letters, 2024, 35(8): 109749-. doi: 10.1016/j.cclet.2024.109749
-
[2]
Shiyi WANG , Chaolong CHEN , Xiangjian KONG , Lansun ZHENG , Lasheng LONG . Polynuclear lanthanide compound [Ce4ⅢCe6Ⅳ(μ3-O)4(μ4-O)4(acac)14(CH3O)6]·2CH3OH for the hydroboration of amides to amine. Chinese Journal of Inorganic Chemistry, 2025, 41(1): 88-96. doi: 10.11862/CJIC.20240342
-
[3]
Jiahong WANG , Zekun XU , Tianjiao LU , Jinming HUANG . Performance of N, Mn doped semi-coke activated carbon catalyzed ozone oxidation for the degradation of tetracycline hydrochloride in water. Chinese Journal of Inorganic Chemistry, 2025, 41(12): 2549-2560. doi: 10.11862/CJIC.20250120
-
[4]
Bharathi Natarajan , Palanisamy Kannan , Longhua Guo . Metallic nanoparticles for visual sensing: Design, mechanism, and application. Chinese Journal of Structural Chemistry, 2024, 43(9): 100349-100349. doi: 10.1016/j.cjsc.2024.100349
-
[5]
Bowen Song , Chenxu Shi , Yinghao Qu , Hongjun Liu , Hui Yang , Xiaoming Wu , Xijun Liu . The electrical properties and charge transport mechanism of MXenes. Chinese Chemical Letters, 2025, 36(6): 110823-. doi: 10.1016/j.cclet.2025.110823
-
[6]
Yuan Dong , Mutian Ma , Zhenyang Jiao , Sheng Han , Likun Xiong , Zhao Deng , Yang Peng . Effect of electrolyte cation-mediated mechanism on electrocatalytic carbon dioxide reduction. Chinese Chemical Letters, 2024, 35(7): 109049-. doi: 10.1016/j.cclet.2023.109049
-
[7]
Hongxia Li , Xiyang Wang , Du Qiao , Jiahao Li , Weiping Zhu , Honglin Li . Mechanism of nanoparticle aggregation in gas-liquid microfluidic mixing. Chinese Chemical Letters, 2024, 35(4): 108747-. doi: 10.1016/j.cclet.2023.108747
-
[8]
Yixin Zhang , Ting Wang , Jixiang Zhang , Pengyu Lu , Neng Shi , Liqiang Zhang , Weiran Zhu , Nongyue He . Formation mechanism for stable system of nanoparticle/protein corona and phospholipid membrane. Chinese Chemical Letters, 2024, 35(4): 108619-. doi: 10.1016/j.cclet.2023.108619
-
[9]
Jia Fu , Shilong Zhang , Lirong Liang , Chunyu Du , Zhenqiang Ye , Guangming Chen . PEDOT-based thermoelectric composites: Preparation, mechanism and applications. Chinese Chemical Letters, 2024, 35(9): 109804-. doi: 10.1016/j.cclet.2024.109804
-
[10]
Ping Wang , Tianbao Zhang , Zhenxing Li . Reconstruction mechanism of Cu surface in CO2 reduction process. Chinese Journal of Structural Chemistry, 2024, 43(8): 100328-100328. doi: 10.1016/j.cjsc.2024.100328
-
[11]
Wenzhong Zhang , Zirui Yan , Lingcheng Chen , Yi Xiao . Sn-fused perylene diimides: Synthesis, mechanism, and properties. Chinese Chemical Letters, 2024, 35(10): 109582-. doi: 10.1016/j.cclet.2024.109582
-
[12]
Hai-Ling Wang , Zhong-Hong Zhu , Hua-Hong Zou . Structure and assembly mechanism of high-nuclear lanthanide-oxo clusters. Chinese Journal of Structural Chemistry, 2024, 43(9): 100372-100372. doi: 10.1016/j.cjsc.2024.100372
-
[13]
Jia-hui Li , Jinkai Qiu , Cheng Lian . Lithium-ion rapid transport mechanism and channel design in solid electrolytes. Chinese Journal of Structural Chemistry, 2025, 44(1): 100381-100381. doi: 10.1016/j.cjsc.2024.100381
-
[14]
Yan Guo , Hongtao Bian , Le Yu , Jiani Ma , Yu Fang . Photochemical reaction mechanism of benzophenone protected guanosine at N7 position. Chinese Chemical Letters, 2025, 36(3): 109971-. doi: 10.1016/j.cclet.2024.109971
-
[15]
Haixia Wu , Kailu Guo . Sulfur reduction reaction mechanism elucidated with in situ Raman spectroscopy. Chinese Chemical Letters, 2025, 36(6): 110654-. doi: 10.1016/j.cclet.2024.110654
-
[16]
Qinyao Jiang , Binhao Wang , Zerui Yan , Sicheng Fan , Dafu Tang , Biwei Xiao , Qiulong Wei . Unraveling the pseudocapacitive sodium-ion storage mechanism of birnessite in organic electrolytes. Chinese Chemical Letters, 2025, 36(11): 110416-. doi: 10.1016/j.cclet.2024.110416
-
[17]
Qiong Su , Chao Hu , Sichan Li , Wenjun Huang , Jianyu Dong , Ren Song , Lan Xu , Guozhao Fang . Sodium-ion batteries at low temperature: Storage mechanism and modification strategies. Chinese Chemical Letters, 2025, 36(12): 111267-. doi: 10.1016/j.cclet.2025.111267
-
[18]
Sanmei Wang , Yong Zhou , Hengxin Fang , Chunyang Nie , Chang Q Sun , Biao Wang . Constant-potential simulation of electrocatalytic N2 reduction over atomic metal-N-graphene catalysts. Chinese Chemical Letters, 2025, 36(3): 110476-. doi: 10.1016/j.cclet.2024.110476
-
[19]
Bingwei Wang , Yihong Ding , Xiao Tian . Benchmarking model chemistry composite calculations for vertical ionization potential of molecular systems. Chinese Chemical Letters, 2025, 36(2): 109721-. doi: 10.1016/j.cclet.2024.109721
-
[20]
Fangwen Peng , Zhen Luo , Yingjin Ma , Haibo Ma . Theoretical study of aromaticity reversal in dimethyldihydropyrene derivatives. Chinese Journal of Structural Chemistry, 2024, 43(5): 100273-100273. doi: 10.1016/j.cjsc.2024.100273
-
[1]
Metrics
- PDF Downloads(2)
- Abstract views(991)
- HTML views(31)

 Login In
Login In




 DownLoad:
DownLoad: