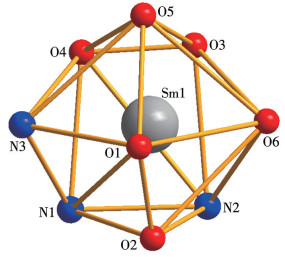
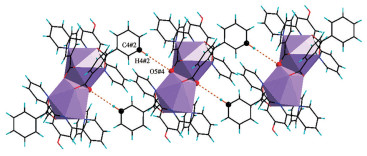
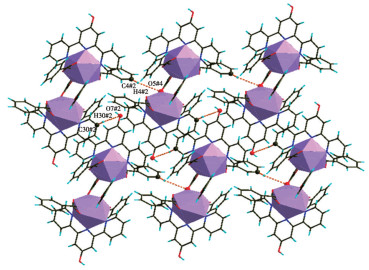
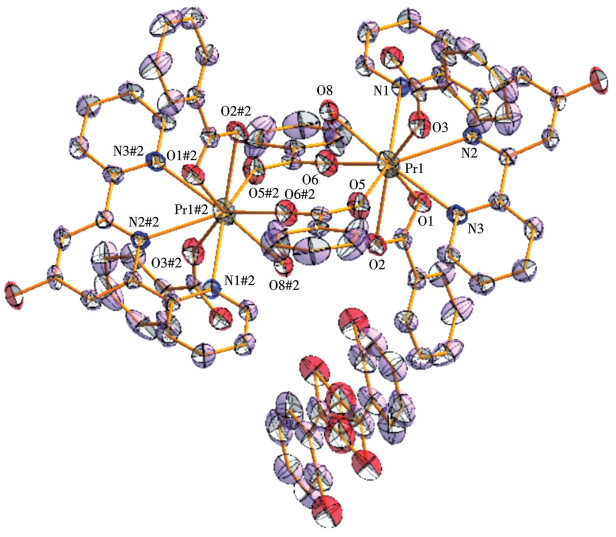
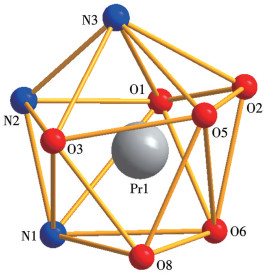
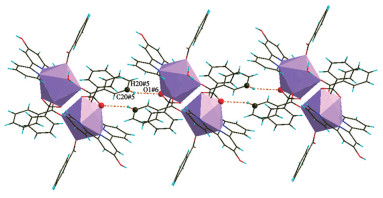
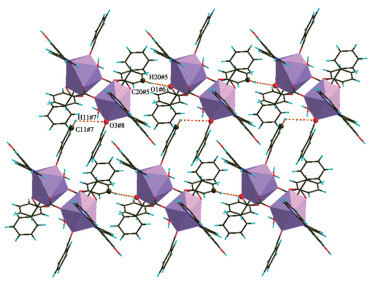
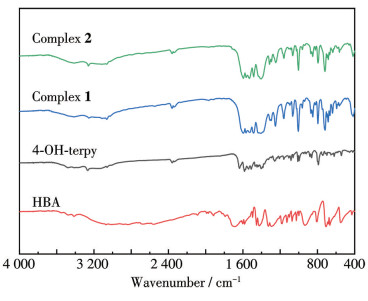
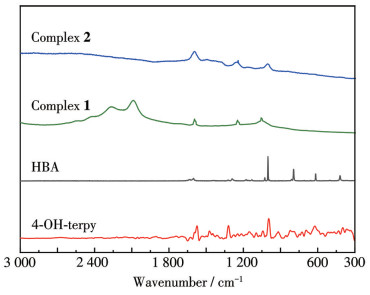
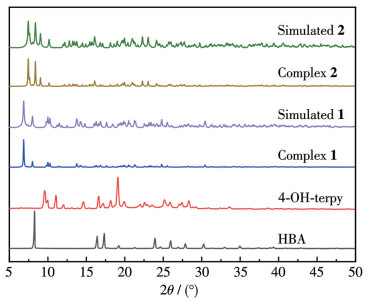
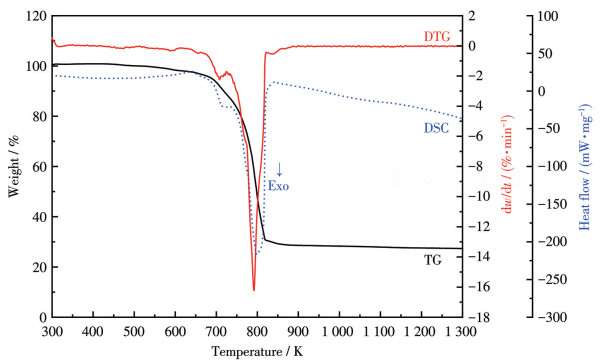
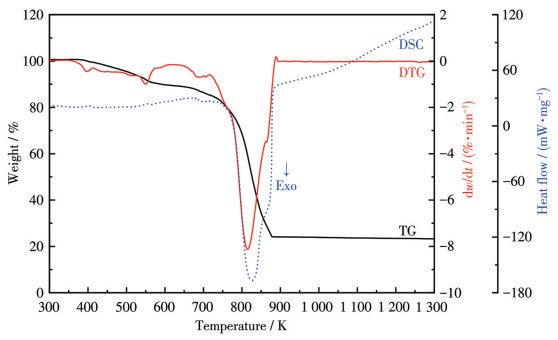
-
[1]
YANG Y L, HU X M, YANG Z, HUANG W. Insights into molecular lanthanide complexes: Construction, properties and bioimaging and biosensing applications[J]. Adv. Funct. Mater.,
2024,21202412970.
-
[2]
ZHONG Y, WU Z N, ZHANG Y, DONG B, BAI X. Circularly polar-ized luminescence of lanthanide complexes: From isolated individu-als, discrete oligomers, to hierarchical assemblies[J]. InfoMat,
2023,5e12392.
doi: 10.1002/inf2.12392
-
[3]
FAN M Y, YU H H, FU P, SU Z M, LI X, HU X L, GAO F W, PAN Q Q. Luminescent Cd(Ⅱ) metal-organic frameworks with anthracene ni-trogen-containing organic ligands as novel multifunctional chemosen-sors for the detection of picric acid, pesticides, and ferric ions[J]. Dyes Pigment.,
2021,185108834.
doi: 10.1016/j.dyepig.2020.108834
-
[4]
TRUPP L, BRUTTOMESSO A C, VARDE M, ELISEEVA S V, RAMIREZ J A, PETOUD S, BARJA B C. Innovative multipodal ligands derived from Troger's bases for the sensitization of lanthanide(Ⅲ) luminescence[J]. Chem.-Eur. J.,
2020,26:16900-16909.
doi: 10.1002/chem.202003524
-
[5]
SHENG Y, JIANG Y J, CHENG Z H, LIU R C, GE J Y, GAO F. Syn-theses, structures, and magnetic properties of acetate-bridged lantha-nide complexes based on a tripodal oxygen ligand[J]. Front. Chem.,
2022,101021358.
doi: 10.3389/fchem.2022.1021358
-
[6]
KOSINSKA-PEZDA M, ZAPALA L, MACIOLEK U, BYCZYNSKI L, WOZNICKA E, ZAPALA W. Thermal study, temperature diffraction patterns and evolved gas analysis during pyrolysis and oxidative de-composition of novel ternary complexes of light lanthanides with mefe-namic acid and 1, 10-phenanthroline[J]. J. Anal. Appl. Pyrolysis,
2021,159105293.
doi: 10.1016/j.jaap.2021.105293
-
[7]
ROMANENKO G V, FOKIN S V, LETYAGIN G A, BOGOMYAKOV A S, OVCHARENKOV I. Structure of lanthanide semiquinolates with nitrogen-containing ligands[J]. J. Struct. Chem.,
2020,61:1594-1598.
doi: 10.1134/S002247662010011X
-
[8]
KHANAGWAL J, KHATKAR S P, DHANKHAR P, BALA M, KUMAR R, BOORA P, TAXAK V B. Synthesis and photolumines-cence analysis of europium(Ⅲ) complexes with pyrazole acid and nitro-gen containing auxiliary ligands[J]. Spectr. Lett.,
2020,53:625-647.
doi: 10.1080/00387010.2020.1817093
-
[9]
WANG C L, SU S L, REN N, ZHANG J J. Construction, thermochem-istry, and fluorescence properties of novel lanthanide complexes syn-thesized from halogenated aromatic carboxylic acids and nitrogen-con-taining ligands[J]. Acta Phys.-Chim. Sin.,
2023,392206035.
-
[10]
NIELSEN L G, JUNKER A K R, SORENSEN T J. Composed in the f-block: Solution structure and function of kinetically inert lantha-nide(Ⅲ) complexes[J]. Dalton Trans.,
2018,47:10360-10376.
doi: 10.1039/C8DT01501E
-
[11]
YANG X P, JONES R A, HUANG S M. Luminescent 4f and d-4f polynuclear complexes and coordination polymers with flexible salen-type ligands[J]. Coord. Chem. Rev.,
2014,273:63-75.
-
[12]
HAYASHI J, SHOJI S, KITAGAWA Y, HASEGAWA Y. Amor-phous lanthanide complexes for organic luminescent materials[J]. Coord. Chem. Rev.,
2022,467214607.
doi: 10.1016/j.ccr.2022.214607
-
[13]
ZHANG L Y, DOU W, LIU W, XU C, JIANG H E, CHEN C Y, GUO L R, TANG X L, LIU W S. Lanthanide complexes with a biphospho-nate ester ligand and their fluorescent properties[J]. Inorg. Chem. Commun.,
2015,59:53-56.
doi: 10.1016/j.inoche.2015.06.024
-
[14]
MA J H, YANG D Q, SONG X F, WANG Y G. Luminescent materi-als of covalent grafting lanthanide complexes to the synthetic clays[J]. J. Lumin.,
2019,212:126-132.
doi: 10.1016/j.jlumin.2019.04.024
-
[15]
LI H R, CHENG W J, WANG Y, LIU B Y, ZHANG W J, ZHANG H J. Surface modification and functionalization of microporous hybrid material for luminescence sensing[J]. Chem.-Eur. J.,
2010,16:2125-2130.
doi: 10.1002/chem.200901687
-
[16]
LI X, LIU Y H, CHI X W, ZHU G Z, GAO F. Synthesis, structures, and magnetic properties of zigzag tetranuclear lanthanide complexes[J]. Z. Anorg. Allg. Chem.,
2020,646:1292-1296.
doi: 10.1002/zaac.202000235
-
[17]
SONG F, ZHANG Y, CHEN J H, HAN T, CHENG P. Syntheses, crystal structures and magnetic properties of three lanthanide-nitronyl nitroxide complexes[J]. J. Rare Earths,
2017,35:24-27.
doi: 10.1016/S1002-0721(16)60168-0
-
[18]
FENG X, LIU L, WANG L Y, SONG H L, SHI Z Q, WU X H, NG S W. Lanthanide coordination polymers based on multi-donor ligand containing pyridine and phthalate moieties: Structures, lumines-cence and magnetic properties[J]. J. Solid State Chem.,
2013,206:277-285.
doi: 10.1016/j.jssc.2013.08.029
-
[19]
HUANG X D, WEN G H, BAO S S, JIA J G, ZHENG L M. Thermo-and light-triggered reversible interconversion of dysprosium-anthracene complexes and their responsive optical, magnetic and dielectric properties[J]. Chem. Sci.,
2021,12:929-937.
doi: 10.1039/D0SC04851H
-
[20]
ZHANG F H, WANG Y Y, LV C, LI Y C, ZHAO X Q. Luminescent complexes associated with isonicotinic acid[J]. J. Lumin.,
2019,207:561-570.
doi: 10.1016/j.jlumin.2018.11.051
-
[21]
SONG X Q, WANG L, ZHENG Q F, LIU W S. Synthesis, crystal structure and luminescence properties of lanthanide complexes with a new semirigid bridging furfurylsalicylamide ligand[J]. Inorg. Chim. Acta,
2012,391:171-178.
doi: 10.1016/j.ica.2012.04.007
-
[22]
DOLOMANOV O V, BOURHIS L J, GILDEA R J, HOWARD J A K, PUSCHMANN H. OLEX2:A complete structure solution, refine-ment and analysis program[J]. J. Appl. Crystallogr.,
2009,42:339-341.
doi: 10.1107/S0021889808042726
-
[23]
SUNDARESWARAN T, JAGAN R, KARTHIKEYAN N, BOAZ B M. Rational analysis of hydrogen bonding interaction in phenazine, 2-hydroxynaphthalene (1:1) cocrystal: From molecular modeling to photophysical properties[J]. J. Mol. Model.,
2024,30351.
doi: 10.1007/s00894-024-06128-3
-
[24]
DU D D, HAO Y F, WANG X X, ZHAO J J, REN N, ZHANG J J. Crystal structure, spectra, and thermal behavior of lanthanide com-plexes with 2-chloro-4-fluorobenzoic acid and 5, 5'-dimethyl-2, 2'-bipyridine[J]. Chinese J. Inorg. Chem.,
2023,39(9):1807-1816.
-
[25]
CEPEDA J, BALDA R, BEOBIDE G, CASTILLO O, FERNÁNDEZ J, LUQUE A, PÉREZ-YÁÑEZ S, ROMÁN P, VALLEJO-SÁNCHEZ D. Lanthanide(Ⅲ)/pyrimidine-4, 6-dicarboxylate/oxalate extended frameworks: A detailed study based on the lanthanide contraction and temperature effects[J]. Inorg. Chem.,
2011,50:8437-8451.
doi: 10.1021/ic201013v
-
[26]
LELLI M, DI BARI L. Solution structure and structural rearrange-ment in chiral dimeric ytterbium(Ⅲ) complexes determined by para-magnetic NMR and NIR-CD[J]. Dalton Trans.,
2019,48:882-890.
doi: 10.1039/C8DT03090A
-
[27]
HE S M, SUN S J, ZHENG J R, ZHANG J J. Molecular spectrum of lanthanide complexes with 2, 3-dichlorobenzoic acid and 2, 2-bipyri-dine[J]. Spectroc. Acta Pt. A -Molec. Biomolec. Spectr.,
2014,123:211-215.
doi: 10.1016/j.saa.2013.12.023
-
[28]
BABU S V, ESWARAMMA S, RAO K. Synthesis, characterization, luminescence and biological activities of lanthanide complexes with a hydrazone ligand[J]. Main Group Chem.,
2018,17:99-110.
doi: 10.3233/MGC-180251
-
[29]
HAO Y F, XU S L, ZHAO J J, GAO J, REN N, ZHANG J J. New nitrogen-containing lanthanide complexes with novel structural, ther-mal, and fluorescence properties[J]. J. Saudi Chem. Soc.,
2024,28101792.
doi: 10.1016/j.jscs.2023.101792
-
[30]
CHEN M H, LIANG B, GUO Y H, LI C F, HE X, HU J H, LI R K, ZENG K, YANG G. Pyrolysis mechanism of polyimide containing bio-molecule adenine building block[J]. Polym. Degrad. Stabil.,
2020,175109124.
doi: 10.1016/j.polymdegradstab.2020.109124
-
[31]
LIU B, LI Y M, WU S B, LI Y H, DENG S S, XIA Z L. Pyrolysis characteristic of tobacco stem studied by Py-GC/MS, TG-FTIR, and TG-MS[J]. BioResources,
2013,8:220-230.
-
[32]
SARIOGLU A O, KAHRAMAN D T, KÖSE A, SÖNMEZ M. Synthe-sis, characterization, photoluminescence properties and cytotoxic activities of Sm(Ⅲ) complexes of β-diketones[J]. J. Mol. Struct.,
2022,1260132786.
doi: 10.1016/j.molstruc.2022.132786
-
[33]
QIU S H, XIAO Q Q, TANG H, XIE Q. First-principles study on electronic structure, optical and magnetic properties of rare earth elements X (X=Sc, Y, La, Ce, Eu) doped with two-dimensional GaSe[J]. Chinese J. Inorg. Chem.,
2024,40(11):2250-2258.
-
[34]
WANG C L, ZHANG J Y, LI X Y, REN N, ZHANG J J. Crystal structure, thermodynamic behavior, and luminescence properties of a new series of lanthanide halogenated aromatic carboxylic acid com-plexes[J]. Arab. J. Chem.,
2022,15104089.

 Login In
Login In






 DownLoad:
DownLoad: