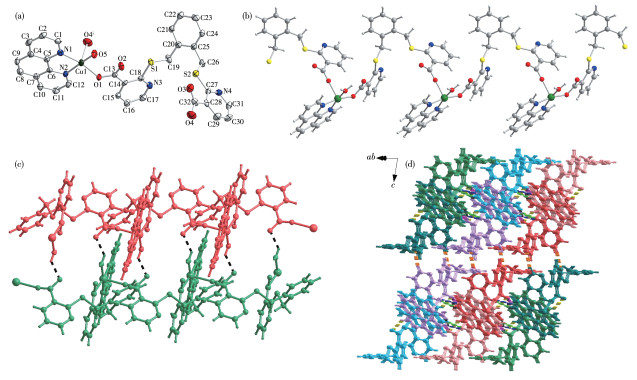
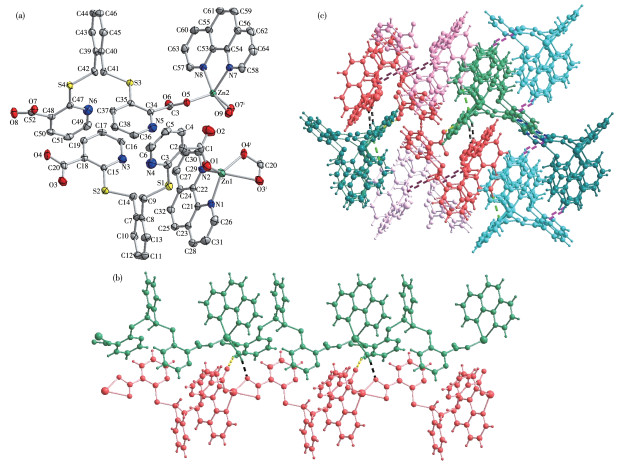
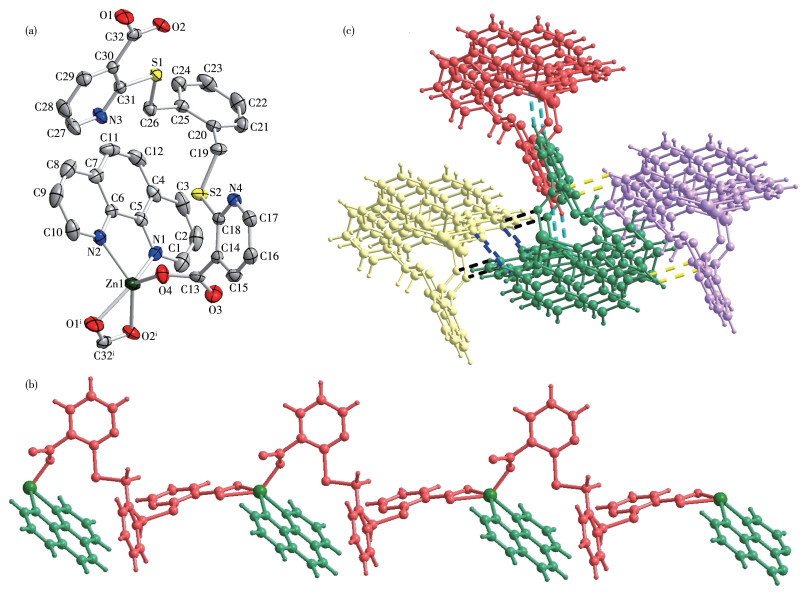
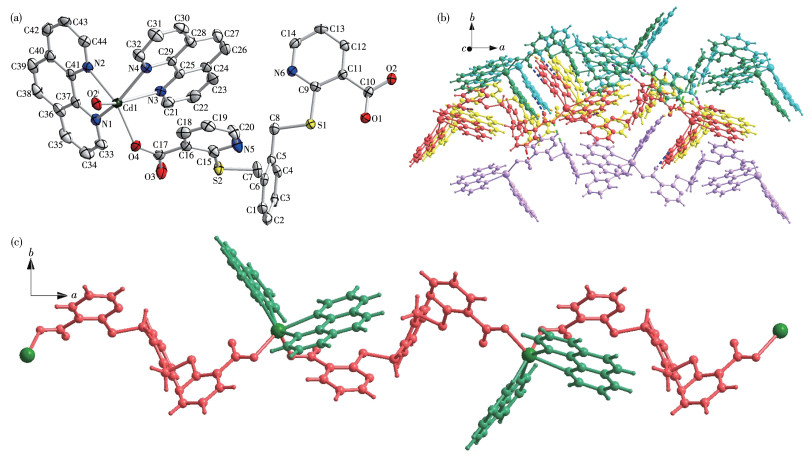
-
[1]
Notash B, Rodbari M F, Gallo G, Dinnebier R. Humidity-induced structural transformation in pseudopolymorph coordination polymers[J]. Inorg. Chem.,
2021,60(12):9212-9223.
doi: 10.1021/acs.inorgchem.1c01360
-
[2]
Jin M, Ando R, Ito H. Distinct fold-mode formation of crystalline Cu(Ⅰ) helical coordination polymers with alternation of the solid-state emission using shape of the counter anions[J]. Inorg. Chem.,
2022,61(1):3-9.
doi: 10.1021/acs.inorgchem.1c02725
-
[3]
Thomas B, Chang B S, Chang J J, Thuo M, Rossini A J. Solid-state nuclear magnetic resonance spectroscopy-assisted structure determination of coordination polymers[J]. Chem. Mater.,
2022,34(17):7678-7691.
doi: 10.1021/acs.chemmater.2c00593
-
[4]
Deng Y F, Wang Y N, Zhao X H, Zhang Y Z. Desolvation-solvation-induced reversible on-off switching of two memory channels in a cobalt(Ⅱ) coordination polymer: Overlay of spin crossover and structural phase transition[J]. CCS Chem.,
2022,4(9):3064-3075.
doi: 10.31635/ccschem.021.202101407
-
[5]
Hu J X, Zhu H L, Meng Y S, Pang J D, Li N, Liu T, Bu X H. Ligand modified and light switched on/off single-chain magnets of {Fe2Co} coordination polymers via metal-to-metal charge transfer[J]. CCS Chem.,
2022,5(4):865-875.
-
[6]
Sheng K, Fan L M, Tian X F, Gupta R K, Tung C H, Sun D. Temperature-induced Sn(Ⅱ) supramolecular isomeric frameworks as promising heterogeneous catalysts for cyanosilylation of aldehydes[J]. Sci. China Chem.,
2020,63:182-186.
doi: 10.1007/s11426-019-9621-x
-
[7]
Chakraborty G, Park I H, Medishetty R, Vittal J J. Two-dimensional metal-organic framework materials: Synthesis, structures, properties and applications[J]. Chem. Rev.,
2021,121(7):3751-3891.
doi: 10.1021/acs.chemrev.0c01049
-
[8]
Lippert B, Sanz P J M. Metallatriangles and metallasquares: The diversity behind structurally characterized examples and the crucial role of ligand symmetry[J]. Chem. Soc. Rev.,
2011,40(9):4475-4487.
doi: 10.1039/c1cs15090a
-
[9]
Lin Z J, Lü J, Hong M C, Cao R. Metal-organic frameworks based on flexible ligands (FL-MOFs): Structures and applications[J]. Chem. Soc. Rev.,
2014,43(16):5867-5895.
doi: 10.1039/C3CS60483G
-
[10]
Ji Z Y, Fan Y R, Wu M Y, Hong M C. A flexible microporous framework with temperature-dependent gate-opening behaviours for C2 gases[J]. Chem. Commun.,
2021,57(31):3785-3788.
doi: 10.1039/D1CC00014D
-
[11]
Chen K F, Mousavi S H, Singh R, Snurr R Q, Li G, Webley P A. Gating effect for gas adsorption in microporous materials-mechanisms and applications[J]. Chem. Soc. Rev.,
2022,51(3):1139-1166.
doi: 10.1039/D1CS00822F
-
[12]
Dai F N, Dou J M, He H Y, Zhao X L, Sun D F. Self-assembly of metal-organic supramolecules: From a metallamacrocycle and a metal-organic coordination cage to 1D or 2D coordination polymers based on flexible dicarboxylate ligands[J]. Inorg. Chem.,
2010,49(9):4117-4124.
doi: 10.1021/ic902178c
-
[13]
Zhang M X, Zhou W, Pham T, Forrest K A, Liu W L, He Y B, Wu H, Yildirim T, Chen B L, Space B, Pan Y, Zaworotko M J, Bai J F. Fine tuning of MOF-505 analogues to reduce low-pressure methane uptake and enhance methane working capacity[J]. Angew. Chem. Int. Ed.,
2017,56(38):11426-11430.
doi: 10.1002/anie.201704974
-
[14]
Zhang M X, Forrest K A, Liu P H, Dang R, Cui H H, Qin G P, Pham T, Tang Y F, Wang S. Significantly enhanced carbon dioxide selective adsorption via gradual acylamide truncation in MOFs: Experimental and theoretical research[J]. Inorg. Chem.,
2022,61(49):19944-19950.
doi: 10.1021/acs.inorgchem.2c03217
-
[15]
Patra R, Titi H M, Goldberg I. Coordination polymers of flexible poly-carboxylic acids with metal ions. Ⅳ: Syntheses, structures, and magnetic properties of polymeric networks of 5-(3, 5)-(dicarboxybenzyloxy)isophthalic acid with Cd(Ⅱ), Cu(Ⅱ), Co(Ⅱ) and Mn(Ⅱ) ions[J]. CrystEngComm,
2013,15:2853-2862.
doi: 10.1039/c3ce27006h
-
[16]
Ma M L, Jia C, Zang S Q. Syntheses, structures, tunable emission and white light emitting Eu3+ and Tb3+ doped lanthanide metal-organic framework materials[J]. Dalton Trans.,
2013,42:10579-10586.
doi: 10.1039/c3dt50315a
-
[17]
Lin Z J, Han L W, Wu D S, Huang Y B, Cao R. Structure versatility of coordination polymers constructed from a semirigid tetracarboxylate ligand: Syntheses, structures, and photoluminescent properties[J]. Cryst. Growth Des.,
2013,13(1):255-263.
doi: 10.1021/cg301405r
-
[18]
Khan S, Frontera A, Matsuda R, Kitagawa S, Mir M H. Topochemical [2+2] cycloaddition in a two-dimensional meta-organic framework via SCSC transformation impacts halogen…halogen interactions[J]. Inorg. Chem.,
2022,61(7):3029-3032.
doi: 10.1021/acs.inorgchem.2c00128
-
[19]
Biedermann F, Schneider H J. Experimental binding energies in supramolecular complexes[J]. Chem. Rev.,
2016,116(9):5216-5300.
doi: 10.1021/acs.chemrev.5b00583
-
[20]
Nößler M, Hunger D, Neuman N I, Reimann M, Reichert F, Winkler M, Klein J, Bens T, Suntrup L, Demeshko S, Stubbe J, Kaupp M, Slageren J, Sarkar B. Fluorinated click-derived tripodal ligands drive spin crossover in both iron(Ⅱ) and cobalt(Ⅱ) complexes[J]. Dalton Trans.,
2022,51:10507-10517.
doi: 10.1039/D2DT01005D
-
[21]
Reek J N H, de Bruin B, Pullen S, Mooibroek T J, Kluwer A M, Caumes X. Transition metal catalysis controlled by hydrogen bonding in the second coordination sphere[J]. Chem. Rev.,
2022,122(14):12308-12369.
doi: 10.1021/acs.chemrev.1c00862
-
[22]
Egorov P A, Grishanov D A, Medvedev A G, Churakov A V, Mikhaylov A A, Ottenbacher R V, Bryliakov K P, Babak M V, Lev O, Prikhodchenko P V. Organoantimony dihydroperoxides: Synthesis, crystal structures, and hydrogen bonding networks[J]. Inorg. Chem.,
2023,62(25):9912-9923.
doi: 10.1021/acs.inorgchem.3c00929
-
[23]
Rams M, Lohmiller T, Böhme M, Jochim A, Foltyn M, Schnegg A, Plass W, Näther C. Weakening the interchain interactions in one dimensional cobalt(Ⅱ) coordination polymers by preventing intermolecular hydrogen bonding[J]. Inorg. Chem.,
2023,62(26):10420-10430.
doi: 10.1021/acs.inorgchem.3c01324
-
[24]
Cui P P, Liu Y, Zhai H G, Zhu J P, Yan W N, Yang Y M. Two copper-organic frameworks constructed from the flexible dicarboxylic ligands[J]. Chin. J. Struct. Chem.,
2020,39(2):368-374.
-
[25]
CUI P P, SUN Y, ZHA Y, LIU S N, ZHANG M X, CAO J Y, WANG Q, WANG X Q. Synthesis, structural characterization, and fluorescence property of three coordination polymers with dicarboxylate ligands[J]. Chinese J. Inorg. Chem.,
2023,39(12):2358-2366.
doi: 10.11862/CJIC.2023.191
-
[26]
Yang C, Wong W T. Self-assembly of guanidinium hexagonal carboxylate: How many H-bonds and H-bonding pattern between ArCOO- and C(NH2)3?[J]. Chem. Lett.,
2004,33(7):856-857.
doi: 10.1246/cl.2004.856
-
[27]
Yang C, Wong W T, Chen X M, Cui Y D, Yang Y S. Star hexacarboxylate: Synthesis, crystal structure and luminescent properties of its terbium complex[J]. Sci. China Ser. B-Chem.,
2003,46(6):558-566.
doi: 10.1360/03yb0050
-
[28]
Xu L, Wang E B, Peng J, Huang R D. A novel coordination polymer with double chains structure: Hydrothermal syntheses, structures and magnetic properties of [Cu(phen)(H2O)2SO4]n (phen=1, 10-phenanthroline)[J]. Inorg. Chem. Commun.,
2003,6(6):740-743.
doi: 10.1016/S1387-7003(03)00055-8
-
[29]
Kamath A, Mishra D K, Brahman D, Pilet G, Sinha B, Tamang A. Poly[diaquo(1, 10-phenanthroline-κ2N1: N10)(μ2-sulphato-κ2O: O')copper(Ⅱ)]: Hydrothermal synthesis, crystal structure and magnetic properties[J]. RSC Adv.,
2016,6:46030-46036.
doi: 10.1039/C6RA03493D
-
[30]
Burlak P V, Samsonenko D G, Kovalenko K A, Fedin V P. Series of cadmium-organic frameworks based on mixed flexible and rigid ligands: Single-crystal-to-single-crystal transformations, sorption, and luminescence properties[J]. Inorg. Chem.,
2023,62(44):18087-18097.
doi: 10.1021/acs.inorgchem.3c02277
-
[31]
Lin Y Q, Tian X M, Xiong Y, Huang C, Chen D M, Zhu B X. Coordination-driven heterochiral self-assembly: Construction of Cd(Ⅱ) coordination polymers with sorption behaviors for iodine and dyes[J]. Inorg. Chem.,
2023,62(49):19887-19897.
doi: 10.1021/acs.inorgchem.3c01747
-
[32]
Mondal S, Sahoo R, Das M C. pH-stable Zn(Ⅱ) coordination polymer as a multiresponsive turn-on and turn-off fluorescent sensor for aqueous medium detection of Al(Ⅲ) and Cr(Ⅵ) oxo-anions[J]. Inorg. Chem.,
2023,62(34):14124-14133.
doi: 10.1021/acs.inorgchem.3c02435

 Login In
Login In






 DownLoad:
DownLoad: