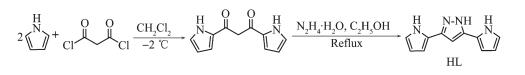
 Scheme 1.
Synthesis route of ligand HL
Scheme 1.
Synthesis route of ligand HL

吡唑双钯(Ⅱ,Ⅱ)配合物的合成与表征及其在Suzuki偶联反应中的催化反应
-
关键词:
- 双钯(Ⅱ)夹子
- / 催化
- / Suzuki偶联反应
English
Pyrazolate-Based Dipalladium (Ⅱ, Ⅱ) Complexes: Syntheses, Characterization and Catalytical Performance in Suzuki-Coupling Reaction
-
Key words:
- dipalladium(Ⅱ) clips
- / catalyst
- / Suzuki-coupling reaction
-
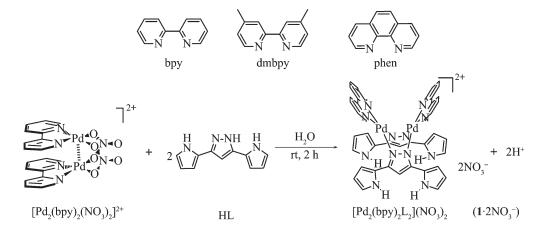
Pyrazolate-based complexes have caught a plenty of attention not only for their structural diversity, such as clusters[1-2], metallomacrocycles[3-7], metal-oraganic cages[8-9], but also for their physico-chemical proper-ties[10-11] and potential application in the field of organocatalysis, biological mimicry, magnetic coupling, electron transfer, opto-electronicmaterials, etc[12]. In our group, we have reported a series of mutifunctional pyrazole-based ligands using as linker and corres-ponding well-defined metallosupramolecular quantita-tively assembled via coordination reaction between pyrazolate linker and dimetal corners [M2(bpy)2(NO3)2](NO3)2 or [M2(phen)2(NO3)2](NO3)2 (M=Pd)[8-9].
Meanwhile, the transition metal palladium complexes have been a hot topic in the area of Suzuki-coupling reaction. Especially, the mononuclear palladium catalysis had played a crucial role in synthetic organic chemistry for Suzuki-coupling reaction. Although mononuclear palladium catalysis has historically dominated the field, the explorations of Pd…Pd bond-containing complexes′ using as catalysts for Suzuki-coupling reaction, are fleetly in progress and become popular for its unique advantages[13-15].
Based on the numerous advantages of pyrazolate-based complexes and the promising catalyst activity of Pd…Pd bond-containing complexes, it is necessary to study the catalytic activity of pyrazolate-based dipalladium(Ⅱ) complexes with weak intramolecular Pd…Pd bonding interaction, as an important and fascinating subclass of palladium coordination.
Herein, we design the solution synthesis of the pyrazolyl ligand HL[16-17] (Scheme 1) and dipalladium(Ⅱ, Ⅱ) clips to get three new Pd…Pd bond-containing clips (Scheme 2), which have been developed and applied into Suzuki-coupling reactions. Excitedly, we find that the Pd…Pd bond-containing clips whose anion have been changed into PF6- show good catalysis properties for Suzuki-coupling reaction although the Pd(Ⅱ) has been coordinated by four inactive N atoms. In addition, all of the complexes have been intensively studied by NMR, ESI-MS, and X-Ray single-crystal diffraction analysis. Interestingly, X-Ray single-crystal diffraction analysis reveals charming structures that the clips not only have weak intramolecular Pd…Pd bonding interaction, but also have chiral axis and F…H hydrogen bond.
1 Experimental
1.1 Materials and instruments
All reagents for synthesis and analysis were obtained commercially with analytical grade and used without further purification and was carried out in the laboratory atmosphere unless reported otherwise. [Pd2(bpy)2L2](PF6)2 (1·2PF6-), [Pd2(dmbpy)2L2](PF6)2 (2·2PF6-), [Pd2(phen)2L2](PF6)2 (3·2PF6-) were prepared according to the literature procedures[18].
1H and 13C NMR spectra were recorded on Bruker AV 400 MHz spectrometers. ESI-MS spectra were recorded on Octant TOF LC-plus 4G mass spectrometer.
1.2 Synthesis and characterization of 1·2PF6-, 2·2PF6- and 3·2PF6-
1.3 X-ray crystallography of complex 1·2PF6-, 2· 2PF6- and 3·2PF6-
The intensity data collections for 1·2PF6-~3·2PF6- (Crystal size / mm: 0.10×0.11×0.15 for complex 1·2PF6-; 0.12×0.18×0.23 for complex 2·2PF6-; 0.11×0.15×0.23 for complex 3·2PF6-) were performed on the Bruker Smart APEX Ⅱ CCD diffractometer equipped with graphite monochromated Mo Kα radiation (λ=0.071 073 nm) at (150±2) K. The empirical absorption corrections were applied by using the SADABS program[19]. The structures were solved by direct methods and refined by full matrix least-squares fitting on F2 by SHELXTL-2014[20]. All non-hydrogen atoms were refined anisotropically. The hydrogen atoms of organic ligands were located geometrically and fixed isotropic thermal parameters. Hydrogen atoms were included in idealized positions with isotropic displacement parameters constrained to 1.5 times the Ueq of their attached carbon atoms for methyl hydrogens, and 1.2 times the Ueq of their attached carbon atoms for all others. SQUEEZE option was used for the final refinement in order to treat the disordered counter anions and solvent molecules.
The details of data with unit cell, space group, data collection, and refinement parameters are prese-nted in Table 1 and Table S1~S3.
CCDC: 1519041, 1·2PF6-; 1536149, 2·2PF6-; 1536150, 3·2PF6-.
1.4 Catalytic activity test
To explore the catalyst activity of pyrazolate-based dipalladium(Ⅱ) complexes with weak intramole-cular Pd…Pd bonding interaction, different reaction conditions have been tried to obtain the feasible solution.
In a typical experiment, the iodobenzene (204 mg, 1 mmol), benzeneboronic acid (136 mg, 1 mmol) and K3PO4 (636 mg, 3 mmol), 1·2PF6- (12 mg, 10 μmol) or 2·2PF6- (12 mg, 10 μmol) or 3·2PF6- (12 mg, 10 μmol)) was added into a 250 mL two-necked flask. Under argon atmosphere, 1, 4-dioxane(100 mL) was added, and the suspension was stirred at 100 ℃. After the reactant disappeared (2 days, monitored the consumption of the starting iodobenzene by TLC), the mixture was cooled to room temperature. The reaction mixture was directly filtered and dried to get crude product (90% purity), than through column chromato-graph eluting with hexane to give the expected comp-ound (141 mg, Yield 88%) as a white solid. 1H NMR (400 MHz, chloroform-d, 298 K, TMS): δ 7.57 (d, J=7.3 Hz, 2H), 7.49 (d, J=8.0 Hz, 2H), 7.42 (t, J=7.6 Hz, 2H), 7.32 (t, J=7.3 Hz, 1H), 7.27~7.23 (m, 2H), 2.39 (s, 3H).
1.2.2 Synthesis and characterization of [Pd2(N^N)2L2]2+-type dimetallo-clips
The self-assembly of bipyrazolate-bridged metallo -clip complex 1·2NO3- was shown in Scheme 2. A mixture of [Pd2(bpy)2(NO3)2](NO3)2 (19.3 mg, 0.025 mmol) with HL (9.9 mg, 0.05 mmol) in water resulted in the formation of 1·2NO3- in quantitative yield. A ten-fold excess of KPF6 was added to the solution to give 1·2PF6- as yellow solid (28.7 mg, Yield 95%). 1H NMR (400 MHz, DMSO-d6, 298 K, TMS): δ 11.36 (s, 4H), 8.64 (s, 4H), 8.32 (s, 4H), 7.98 (s, 4H), 7.55 (s, 4H), 7.20 (s, 4H), 6.88 (d, J=16.8 Hz, 6H), 5.99 (s, 4H). 13C NMR (400 MHz, DMSO-d6, 298K, TMS): δ 156.99, 150.88, 147.14, 142.71, 128.22, 124.89, 122.49, 120.56, 109.31, 108.04. Elemental analysis calculated for C42H34 F12N12P2Pd2(%): C: 41.71; H: 2.83; N: 13.90. Found(%): C: 41.65; H: 2.87; N: 13.84. ESI-MS (CH3CN, m/z): Calcd. for [1·PF6-]+ 1 064.86, Found 1 064.86; [1]2+ 459.95, Found 459.95 (Fig.S2~S4).
The same procedure as employed for 2·2PF6- was followed except that [Pd2(dmbpy)2(NO3)2](NO3)2 (20.7 mg, 0.025 mmol) was used as the starting material to give 2·2PF6- as yellow solid (30.3 mg, Yield 96%). 1H NMR (400 MHz, DMSO-d6, 298 K, TMS): δ 11.34 (s, 4H), 8.52 (s, 4H), 7.82 (d, J=6.0 Hz, 4H), 7.39 (d, J=6.0 Hz, 4H), 7.17 (s, 4H), 6.88 (s, 2H), 6.86 (s, 4H), 6.00 (q, J=2.7 Hz, 4H), 1.06 (s, 12H). 13C NMR (400 MHz, DMSO-d6, 298K, TMS): δ 156.27, 155.16, 150.11, 147.09, 128.62, 125.47, 122.59, 120.52, 109.30, 107.92, 21.45. Elemental analysis calculated for C46H42F12N12P2Pd2(%): C: 43.65; H: 3.34; N: 13.28. Found(%): C: 43.58; H: 3.42; N: 13.11. ESI-MS (CH3CN, m/z): Calcd. for [2·PF6-]+ 1 121.14; Found 1 120.96; [2]2+ 488.07, Found 488.00 (Fig.S5~S7).
The same procedure as employed for 3·2PF6- was followed except that [Pd2(phen)2(NO3)2](NO3)2 (20.5 mg, 0.025 mmol) was used as the starting material to give 3·2PF6- as yellow solid (28.9 mg, Yield 92%). 1H NMR (400 MHz, DMSO-d6, 298 K, TMS): δ 11.43 (d, J=2.9 Hz, 4H), 8.92 (dd, J=8.3, 1.3 Hz, 4H), 8.36 (dd, J=5.4, 1.3 Hz, 4H), 8.25 (s, 4H), 7.88 (dd, J=8.3, 5.4 Hz, 4H), 7.28 (ddd, J=3.9, 2.6, 1.5 Hz, 4H), 7.03 (s, 2H), 6.88 (td, J=2.7, 1.4 Hz, 4H), 5.94 (dt, J=3.6, 2.4 Hz, 4H).13C NMR (400 MHz, DMSO-d6, 298 K, TMS): δ 151.78, 147.48, 141.53, 131.00, 128.18, 126.69, 122.59, 120.61, 109.33, 108.34, 102.33. Elemental analysis calculated for C52H48F12N14O2P2Pd2(%): C: 44.49; H: 3.45; N: 13.97. Found(%): C: 44.37; H: 3.52; N: 13.83. ESI-MS (CH3CN, m/z): Calcd. for [3·PF6-]+ 1 112.08; Found 1 112.87; [3]2+ 483.05, Found 482.96 (Fig.S8~S10).
1.2.1 General pyrazole ligand preparation
The pyrazole ligand, 3, 5-di(2-pyrrolyl)-pyrazole (HL), was synthesized according to a reported procedure (referring to supporting information for the synthesis). 1H NMR (400 MHz, DMSO-d6, 298 K, TMS): δ 12.65 (s, 1H), 11.23 (s, 1H), 11.12 (s, 1H), 6.85 (s, 1H), 6.71 (s, 1H), 6.55 (s, 1H), 6.48 (s, 1H), 6.28 (s, 1H), 6.11 (s, 1H), 6.05 (d, J=3.0 Hz, 1H) (Fig.S1).
2 Results and discussion
2.1 Characterization of [Pd2(N^N)2L2]2+-type dimetallo-clips
Complexes 1·2PF6-, 2·2PF6- and 3·2PF6- were fully characterized by NMR and ESI-MS. The NMR spectrum of 1·2PF6- clearly shows that a 1:1 Pd(bpy) to L-complex was formed (Fig.S2). As shown in 1H NMR of 1·2PF6- (Fig.S2), two N-protons of the ligand in the product turned out to be only one singlet at δ 11.36, which presents two singlets at 11.24 and 11.12 for H6 and H6′, respectively, before self-assembly (see synthesis part). This can only be explained by that the N-protons of the ligand in the complex HL were equivalent. In addition, the resonance signals of the CH of two pyrrole rings changed from a pair single peak into a multiple peak. Moreover, the assignment of product 1·2PF6- as dimetallo-clip [Pd2(N^N)2L2]2+-type dimetallo-clip is further proved by ESI-MS studies, where multiply charged molecular ions corresponding to intact dimetallo-clip were observed. The ESI-MS experiment was performed in acetonitrile solution. As shown in Fig.S4, the multiply charged molecular ions of 1·2PF6- at m/z 1 064.86 and 459.95 are ascribed to the cations of [1·PF6-]+, [1]2+, respectively. The other two similar complexes 2·2PF6- and 3·2PF6- are also obtained and characterized by the same method (Fig.S5~S10). All the characterizitions have demonstrated that the preparaton of these organometallic clips as mentioned is successful. The solid state structures of these "molecular clips" have been further confirmed by single-crystal X-ray diffraction analysis.
2.2 Crystal structures of the dimetallo-clips
Single crystals of 1·2PF6-, 2·2PF6- and 3·2PF6- were obtained by vapor diffusion of diethyl ether into their acetonitrile solutions. Their corner-like structure are strongly supported by single-crystal structure analysis.
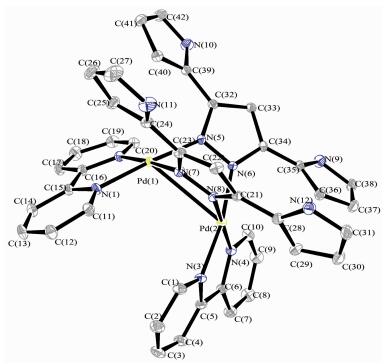
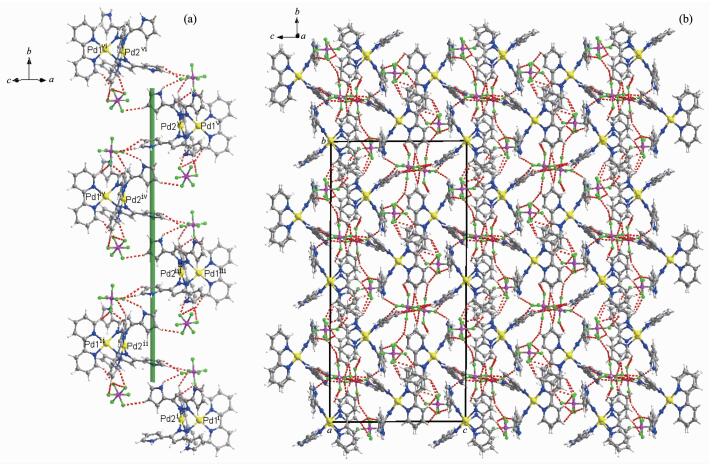
An ORTEP representation of 1·2PF6- is shown in Fig. 1 and selected bond lengths and bond angles are listed in Table S1. The complex 1·2PF6- crystallizes in the monoclinic space group P21/c. The crystal structure analysis for 1·2PF6- reveals a Pd2 dimetallic corner-shaped structure with one (μ-pyrazolato-N, N′)2 doubly bridged [Pd(bpy)]2 dimetal corner. The [Pd2L2]-tpye corner is constituted by two monopyrazole ligands L and two Pd(bpy) motifs. The central six membered ring consisting of the two Pd atoms and the four pyrazolyl N atoms has a boat-shaped conformation with Pd-Npz distances between 0.200 41(18) and 0.202 82(19) nm. The two pyrazole rings form a dihedral angle of 92.14° and form dihedral angles of about 5.65°, 15.71°, 35.89° and 39.53° with pyrrole planes in the pyrazole bridged ligand. The dihedral angle between the two bpy ligands in the corner is 65.53°, which is smaller than the dihedral angle between the pyrazolate ligands. The distance of Pd1-Pd2 is 0.311 79(3) nm which is in the range of typical Pd…Pd interactions (0.26~0.33 nm) [3] and reveals weak Pd…Pd interaction. As shown in Fig. 2, in the solid crystal structure, a 1D helical chain with the pitch of 0.916 nm along the b axis is constructed through multiple intermolecular C-H…F, N-H…F hydrogen bonds between dimetallo-clip cation and counter anions and intermolecular π…π packing interaction between bpy ligands. Further-more, an anionic channel along crystallographic c axis exist in crystal structure. A 2D supramolecular network structure is showed in Fig. 2b.
An ORTEP representation of 2·2PF6- is shown in Fig.S14 and selected bond lengths and bond angles are listed in Table S2. The complex 2·2PF6- crystallizes in the monoclinic space group P21/c. The crystal stru-cture analysis for 2·2PF6- also display a Pd2 dimetallic corner-shaped structure with one (μ-pyrazolato-N, N′)2 doubly bridged [Pd(dmbpy)]2 dimetal corner similar to that of 1·2PF6-. The dihedral angle between the dmbpy ligands in the corner is 67.3°, which are significantly larger than those of 1·2PF6-. The separations of Pd…Pd (0.308 28(8) nm) is comparable to those of 1·2PF6- and also indicate weak Pd…Pd interactions. The two pyrazole rings form a dihedral angle of 67.32° and form dihedral angles of about 3.89°, 6.25°, 10.85° and 37.19° with pyrrole planes in the pyrazole bridged ligand, which are significantly smaller than those of 1·2PF6-. In the crystal, molecules of complex 2·2PF6- pack by multiple intermolecular C-H…F, N-H…F hydrogen bonds between dimetal corner cations and counter anions, intermolecular π…π packing interac-tion and intermolecular C-H…π bonds between neighboring molecules, which built a 1D helical chain with the pitch of 0.946 nm along the b axis and a 2D supramolecular network structure shown in Fig.S15.
An ORTEP representation of 3·2PF6- is shown in Fig.S16 and selected bond lengths and bond angles are listed in Table S3. The complex 3·2PF6- crystall-izes in the monoclinic space group C2/c. As shown in Fig.S16, the crystal structure analysis for 3·2PF6- reveals a Pd2 dimetallic corner-shaped structure with one (μ-pyrazolato-N, N′)2 doubly bridged [Pd(phen)]2 dimetal corner. In the dipalladium corner, the dihedral angles between the two pyrazolate planes is 98.18°, and the two pyrazole rings form dihedral angles of about 2.49°, 2.49 °, 21.05° and 21.05° with pyrrole planes in the pyrazole bridged ligand. The dihedral angle between the phen ligands within the dimetal corners is 51.9°, which is obviously smaller than those of 1·2PF6- and 2·2PF6-. The separation of Pd1…Pd#1 (0.303 00(5) nm) is slightly shorter than the sum of the van der Waals radii of palladium, indicating the presence of weak Pd…Pd interactions. Multiple intermolecular C-H…F, N-H…F hydrogen bonds between dimetal corner cations and counter anions and intermolecular C-H…π bonds between neigh-boring molecules construct a 1D helical chain with the pitch of 0.525 nm along the b axis and a 2D supramolecular network structure is shown in Fig.S17.
2.3 Catalytic activity
We were pleased to find that the reaction proceeded smoothly in refluxing 1, 4-dioxane for 48 h to provide product biphenyl in good yields (Table 2). But it could not be further improved as increasing the ratio of 1·2PF6- (2·2PF6-, or 3·2PF6-).
With the optimal reaction conditions (1:1 of nA:nB, K3PO4, 1%(n/n) catalyst, 1, 4-dioxane, at 90 ℃) in hand, the reactions of A with various boric acid deriv-atives were explored to gauge the generality of this process (Table 3). For the substrates bearing electron-donating (-OMe, -CH3) and electron-withdrawing groups (-CHO) on the phenyl ring (R1, R2), the reactions proc-eeded smoothly to give the corresponding biphenyl compounds C1 in 78%~88% yields, C2 in 60%~78% yields, C3 in 56%~76% yields. In addition, electron-donating (-OMe, -CH3) boric acids tend to have higher yields than electron-withdrawing groups (-CHO) boric acids during the reactions. The reasons of the difference yields of C1 (C2, or C3) may be the different steric effect of 1·2PF6- (2·2PF6- or 3·2PF6-) during the reactions.
3 Conclusions
In summary, of three Pd…Pd bond-containing complexes are synthesized via a directed coordination driving approach that occurs along with spontaneous deprotonation of the ligand and three organometallic "molecular clips" (M2L2) are generated in nearly quantitative yield. All of the structures are characterized by 1H NMR, 13C NMR, ESI-MS, and single-crystal X-ray diffraction analysis. More interestingly, pyrazolate-based dipalladium(Ⅱ) clips with weak intra-molecular Pd…Pd bonding interaction which are coordinated by four inactive N atoms exhibit good catalytic activity for Suzuki-coupling reaction.
-
-
[1]
Ahmed B M, Gellert M. Inorg. Chem., 2016, 55:7717-7728 doi: 10.1021/acs.inorgchem.6b01172
-
[2]
Nicola C D, Garau F, Gazzano M, et al. Cryst. Growth Des., 2015, 15:1259-1272 doi: 10.1021/cg501647r
-
[3]
Yu S Y, Huang H P, Li S H, et al. Inorg. Chem., 2005, 44:9471-9488 doi: 10.1021/ic0509332
-
[4]
Ning G H, Yao L Y, Liu L X, et al. Inorg. Chem., 2010, 49:7783-7792 doi: 10.1021/ic100724r
-
[5]
Ning G H, Xie T Z, Pan Y J, et al. Dalton Trans., 2010, 39:3203-3211 doi: 10.1039/b926138a
-
[6]
Xie T Z, Guo C, Yu S Y, et al. Angew. Chem. Int. Ed., 2012, 51:1177-1181 doi: 10.1002/anie.201106504
-
[7]
Tong J, Yu S Y, Li H. Chem. Commun., 2012, 48:5343-5345 doi: 10.1039/c2cc31234d
-
[8]
Yu S Y, Jiao Q, Li S H, et al. Org. Lett., 2007, 9:1379-1382 doi: 10.1021/ol070286d
-
[9]
Jiang X F, Deng W, Jin R, et al. Dalton Trans., 2014, 43:16015-16024 doi: 10.1039/C4DT01745E
-
[10]
Fujisawa K, Ishikawa Y, Miyashita Y, et al. Inorg. Chim. Acta, 2010, 363:2977-2989 doi: 10.1016/j.ica.2010.05.014
-
[11]
Sun Q F, Liu L X, Huang H P, et al. Inorg. Chem., 2008, 47:2142-2154 doi: 10.1021/ic701344p
-
[12]
La Monica G, Ardizzoia G A. Progress in Inorganic Chemistry: Vol. 46. Karlin K D. Ed. New York: Wiley, 1997: 151-238 doi: 10.1002/9780470166475
-
[13]
Ogura T, Yoshida K, Yanagisawa A, et al. Org. Lett., 2009, 11:2245-2248 doi: 10.1021/ol900533h
-
[14]
Baya M, Houghton J, Konya D, et al. J. Am. Chem. Soc., 2008, 130:10612-10624 doi: 10.1021/ja8012903
-
[15]
Oldenhof S, Lutz M, Bruin B, et al. Organometallics, 2014, 33:7293-7298 doi: 10.1021/om5010622
-
[16]
Hassner A, Fogler E. Heterocycles, 2009, 77:301-309 doi: 10.3987/COM-08-S(F)15
-
[17]
Huang H P, Li S H, Yu S Y, et al. Inorg. Chem. Commun., 2005, 8:656-660 doi: 10.1016/j.inoche.2005.04.011
-
[18]
胡佳华, 邓威, 蒋选丰, 等.无机化学学报, 2015, 31:1278-1286 http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?file_no=20150704&flag=1HU Jia-Hua, DENG Wei, JIANG Xuan-Feng, et al. Chinese J. Inorg. Chem., 2015, 31:1278-1286 http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?file_no=20150704&flag=1
-
[19]
Sheldrick G M. SADABS, University of Göttingen, Germany, 1996.
-
[20]
Sheldrick G M. SHELXTL-2014, Madison, Wisconsin, USA: Siemens Analytical X-ray Division, 2014.
-
[1]
-
Table 1. Crystal structure determination data for complexes 1·2PF6-, 2·2PF6- and 3·2PF6-

Table 2. Catalytic activity of complexes 1·2PF6-, 2·2PF6- and 3·2PF6-

Table 3. Catalytic activity of complexes 1·2PF6-, 2·2PF6- and 3·2PF6-

-

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 4
- 文章访问数: 944
- HTML全文浏览量: 89




 下载:
下载:





 下载:
下载:

