
基于3, 5-双 (4-吡啶基)-吡啶的两个钴 (Ⅱ) 配合物的合成与晶体结构
English
Syntheses and Crystal Structures of Two Cobalt (Ⅱ) Compounds Based on 3, 5-Bis (4-pyridyl)-pyridine
-
Key words:
- crystal structure
- / synthesis
- / Co (Ⅱ) compound
- / N-containing ligands
-
Crystal engineering of coordination polymers has attracted intensive attention due to their variable archite-ctures and potential applications in optical materials[1-3], catalysis[4-5], gas adsorption[6], magnetism[7-8], etc. A large number of coordination polymers (CPs) of N-containing ligands and carboxylic acids have been reported[9-12], not only because they can incorporate virtues of different functional groups and it is easier to get architecture controlled by changing one of the above two kinds of ligands, but also due to allowing free rotation of the imidazole ring to meet the requirement of coordination geometries of metalions[13-14]. To date, however, how to rationally design and synthesize the desired architectures and properties is still a great challenge because the formation of CPs may be easily affected by many factors.
Considering that mixed-ligand of N-containing ligands and carboxylic acids offers greater tunability of the structural framework, two carboxylate ligands of trans-1, 4-cyclohexanedicarboxylic acid (trans-H2chdc) and 4, 4'-oxydibenzoic acid (H2oba) were introduced to react with 3, 5-bis (4-pyridyl)-pyridine (BPYP) of V-shaped flexible polypyridyl ligand and cobalt nitrate. Then two new compounds [Co (BPYPY)2(H2O)4]·(trans-chdc)·4H2O (1) and {[Co (BPYPY)(H2O)4]·(oba)}n (2) were obtained. Further, we described their syntheses and crystal structures in detail.
1 Experimental
1.1 Materials and measurement
All the chemicals were commercially purchased and used without further purification. Elemental analyses were performed on an Elementar Vario MICRO Elemental Analyzer. Single crystal X-ray diffraction measurements were carried out on a Bruker Smart APEX Ⅱ CCD diffraction.
1.2 Synthesis of compounds
[Co (BPYPY)2(H2O)4]·(trans-chdc)·4H2O (1): A mixture of Co (NO3)2·6H2O (29.1 mg, 0.1 mmol), trans-H2chdc (17.2 mg, 0.1 mmol) and BPYPY (23.3 mg, 0.1 mmol) were dissolved in 6 mL of H2O/DMF (2:4, V/V). The final mixture was placed in a Parr Teflon-lined stainless steel vessel (15 mL) and heated at 90 ℃ for 3 days. After being cooled to room temperature, orange block crystals were obtained. The yield was ca. 63% based on BPYPY ligand. Anal. Calcd. for C38H48CoN6O12(%): C, 54.30; H, 5.72; N, 10.00. Found (%): C, 54.27; H, 5.74; N, 9.88.
{[Co (BPYPY)(H2O)4]·(oba)}n (2): A mixture of Co (NO3)2·6H2O (29.1 mg, 0.1 mmol), H2oba (25.8 mg, 0.1 mmol) and BPYPY (23.3 mg, 0.1 mmol) were dissolved in 6 mL of H2O/DMF (2:4, V/V). The final mixture was placed in a Parr Teflon-lined stainless steel vessel (15 mL) and heated at 95 ℃ for 3 days. Orange block crystals were collected. The yield was ca. 60% based on BPYPY ligand. Anal. Calcd. for C29H27CoN3O9(%): C, 56.09; H, 4.35; N, 6.77. Found (%): C, 56.07; H, 4.33; N, 6.79.
1.3 Structure determination of Single Crystals
The regular crystals of compounds 1 and 2 were mounted on a Bruker Smart APEX Ⅱ CCD diffraction (λ=0.071 073 nm). In all cases, empirical absorption corrections by SADABS were applied to the intensity data[15]. The structures were solved by direct methods, and non-hydrogen atoms were refined anisotropically on F2 by the full-matrix least-squares techniques using SHELXL-97 program package[16]. Crystallographic data and structure refinements of compounds 1 and 2 are listed in Table 1. The selected bond lengths and bond angles of compounds are listed in Table 2.
Compound 1 2 Empirical formula C38H48CoN6O12 C29H27CoN3O9 Formula weight 839.75 620.47 Crystal system Monoclinic Orthorhombic Space group P21/n Pccn a / nm 1.479 47(6) 0.800 81(2) b/ nm 0.692 80(3) 2.700 75(7) c / nm 1.954 89(8) 1.230 98(3) β/(°) 99.280 0(10) V/ nm3 1.977 49(14) 2.662 35(12) Z 2 4 Dc/(g·cm-3) 1.410 1.548 Size / mm 0.28×0.26×0.22 0.30×0.28×0.26 μ/ mm-1 0.504 0.708 F(000) 882 1284 θmin, θmax / (°) 2.71, 30.67 2.65, 27.56 Goodness of fit on F2 1.033 1.058 Reflections collected 13 752 13 759 Independent reflections (Rint) 4 892(0.021 1) 3048(0.017 7) R1, wR2 [I > 2σ(I)] 0.033 7, 0.086 8 0.030 7, 0.084 9 R1, wR2 (all data) 0.045 2, 0.093 8 0.035 7, 0.088 5 (Δρ) max, (Δρ) min/ (e·nm-3) 413, -298 272, -349 Compound 1 Co(1)-O(1) 0.207 40(11) Co(1)-N(1) 0.218 06(12) Co(1)-O(2) 0.208 54(11) O(1)_Co(1)-O(1)#1 180.00(7) O(1)-Co(1)-O(2) 88.61(5) O(1)#1-Co(1)-O(2) 91.39(5) O(1)-Co(1)-O(2)#1 91.39(5) O(2)-Co(1)-O(2)#1 180.00(8) O(2)-Co(1)-N(1)#1 91.04(4) O(1)-Co(1)-N(1)#1 87.54(5) O(1)-Co(1)-N(1) 92.46(5) O(1)#1-Co(1)-N(1) 87.54(5) N(1)-Co(1)-O(2) 88.96(4) O(2)#1-Co(1)-N(1) 91.04(4) N(1)#1-Co(1)-N(1) 180.00(16) Compound 2 Co(1)-N(1) 0.216 16(11) Co(1)-O(1) 0.209 37(11) Co(1)-O(2) 0.213 65(11) O(2)-Co(1)-N(1) 89.77(4) O(1)-Co(1)-O(2)#2 93.38(4) O(2)-Co(1)-O(2)#2 180 O(1)#2-Co(1)-O(1) 180 O(1)-Co(1)-O(2) 86.62(4) N(1)#2-Co(1)-N(1) 180.00(3) O(1)-Co(1)-N(1) 87.11(4) O(2)#2-Co(1)-N(1) 90.23(4) O(1)#2-Co(1)-N(1) 92.89(4) Symmetry codes: #1:-x+1, -y+1, -z for 1; #2:-x+1, -y, -z+1 for 2. CCDC: 1437803, 1; 1401543, 2.
2 Result and discussion
2.1 Crystal structure of compound 1
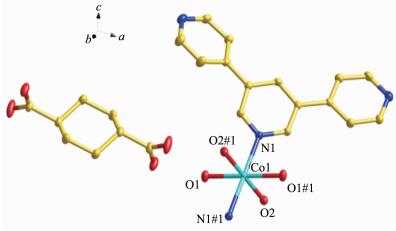
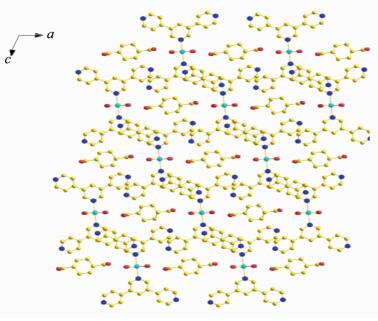
Compound 1 crystallizes in the monoclinic crystal system with P21/n space group. The asymmetric unit consists of half of a Co atom, one BPYPY ligand, two coordinated water molecules and half of a free trans-chdc2-, two free water molecules. As shown in Fig. 1, each Co (Ⅱ) atom is six coordinated by two nitrogen atoms from two BPYPY ligands and four oxygen atoms from four coordinated water molecules, to form the nuclear of [Co (BPYPY)2(H2O)4]. The bond distances of Co-N are 0.218 06(12) nm. The Co-O (1) bond length is 0.207 40(11) nm, and Co-O (2) bond length is 0.208 54(11) nm. It is noteworthy that [Co (BPYPY)2(H2O)4] cross-link trans-chdc2- by O-H…O hydrogen bonds generating the 2D network (Fig. 2).
2.2 Crystal structure of compound 2
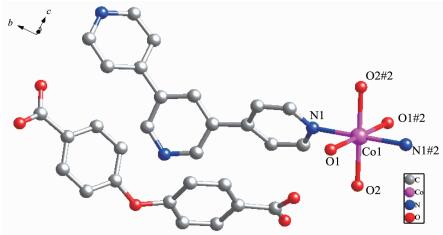
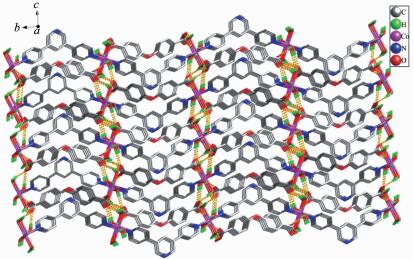
Compound 2 crystallizes in the orthorhombic crystal system with the space group of Pccn, and the asymmetric unit consists of half of a Co atom, half of a BPYPY ligand, two coordinated water molecules, half of a free completely deprotonated oba2- ligand. Each Co (Ⅱ) atom is surrounded by two nitrogen atoms from two BPYPY ligands, and four oxygen atoms from four coordinated water molecules, adopting an octahedral geometry (Fig. 3). The bond distance of Co-N is 0.216 16(11) nm. The Co-O bond lengths are 0.209 37(11) and 0.213 65(11) nm, respectively. The 1D linear chain with a Co…Co distance of 1.408 49 nm and a Co…Co…Co angle of 180.00° is generated by BPYPY ligands and the Co (Ⅱ) cations. Then, the 1D linear chains are held together via O-H…O interaction to generate an infinite 3D network (Fig. 4).
3 Conclusions
In summary, by using the V-shaped ligand, 3, 5-bis (4-pyridyl)-pyridine (BPYPY), and two kinds of dicarboxylates, trans-1, 4'-cyclohexanedicarboxylic acid (trans-chdc) and 4, 4'-oxydibenzoic acid (H2oba), we have synthesized two new compounds in mild hydrothermal method. Compound 1 has a 2D network generated by hydrogen bond cross-linked [Co (BPYPY)2(H2O)4] and trans-chdc2-. Compound 2 is a 1D chain and further extended via O-H…O interaction to generate infinite 3D supramolecular structure.
-
-
[1]
Hu Z, Deibet B J, Li J. Chem. Soc. Rev., 2014, 43:5815-5840 doi: 10.1039/C4CS00010B
-
[2]
Zhao X, Wang X, Wang S, et al. Cryst. Growth Des., 2012, 12:2736-2739 doi: 10.1021/cg3002866
-
[3]
Liu X G, Wang H, Chen B, et al. Chem. Commun., 2015, 51: 1677-1680 doi: 10.1039/C4CC08945F
-
[4]
Wang L H, Zeng Y, Shen A G, et al. Chem. Commun., 2015, 51:2052-2055 doi: 10.1039/C4CC08089K
-
[5]
Roberts J M, Fini B M, Sarjeant A A, et al. J. Am. Chem. Soc., 2012, 134:3334-3337 doi: 10.1021/ja2108118
-
[6]
OKeeffe M, Yaghi O M. Chem. Rev., 2012, 112:675-702 doi: 10.1021/cr200205j
-
[7]
张春丽, 覃玲, 杨清香, 等.无机化学学报, 2013, 29(10):2157-2161 http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?flag=1&file_no=20131021&journal_id=wjhxxbcnZHANG Chun-Li, QIN Lin, YANG Qing-Xiang, et al. Chinese J. Inorg. Chem., 2013, 29(10):2157-2161 http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?flag=1&file_no=20131021&journal_id=wjhxxbcn
-
[8]
Uemura K, Yamasaki Y, Komagawa Y, et al. Angew. Chem. Int. Ed., 2007, 46:6662-6665 doi: 10.1002/(ISSN)1521-3773
-
[9]
Qin L, Ju Z M, Wang Z J, et al. Cryst. Growth Des., 2014, 14:2742-2746 doi: 10.1021/cg500269h
-
[10]
Qin L, Hu J S, Zhang M D, et al. Chem. Commun., 2012, 48: 10757-10759 doi: 10.1039/c2cc36003a
-
[11]
Zhang C L, Qin L, Zheng H G. Inorg. Chem. Commun., 2013, 36:192-194 doi: 10.1016/j.inoche.2013.09.014
-
[12]
Zhang M D, Di C M, Qin L, et al. Cryst. Growth Des., 2012, 12:3957-3963 doi: 10.1021/cg300386p
-
[13]
Zhang C L, Li Y L, Wang T, et al. Chem. Commun., 2015, 51:8338-8341 doi: 10.1039/C5CC01072A
-
[14]
Shi Z Q, Guo Z J, Zheng H G. Chem. Commun., 2015, 51: 8300-8303 doi: 10.1039/C5CC00987A
-
[15]
SMART and SAINT, Siemens Analytical X-ray Instrument Inc. , Madison, WI, 1996.
-
[16]
Sheldrick G M. SHELXL-97, Program for the Refinement of Crystal Structure, Universirty of Göttingen, Germany, 1997.
-
[1]
-
Table 1. Crystallographic data for compounds 1 and 2
Compound 1 2 Empirical formula C38H48CoN6O12 C29H27CoN3O9 Formula weight 839.75 620.47 Crystal system Monoclinic Orthorhombic Space group P21/n Pccn a / nm 1.479 47(6) 0.800 81(2) b/ nm 0.692 80(3) 2.700 75(7) c / nm 1.954 89(8) 1.230 98(3) β/(°) 99.280 0(10) V/ nm3 1.977 49(14) 2.662 35(12) Z 2 4 Dc/(g·cm-3) 1.410 1.548 Size / mm 0.28×0.26×0.22 0.30×0.28×0.26 μ/ mm-1 0.504 0.708 F(000) 882 1284 θmin, θmax / (°) 2.71, 30.67 2.65, 27.56 Goodness of fit on F2 1.033 1.058 Reflections collected 13 752 13 759 Independent reflections (Rint) 4 892(0.021 1) 3048(0.017 7) R1, wR2 [I > 2σ(I)] 0.033 7, 0.086 8 0.030 7, 0.084 9 R1, wR2 (all data) 0.045 2, 0.093 8 0.035 7, 0.088 5 (Δρ) max, (Δρ) min/ (e·nm-3) 413, -298 272, -349 Table 2. Selected bond lengths(nm) and bond angles(°) of compounds 1 and 2
Compound 1 Co(1)-O(1) 0.207 40(11) Co(1)-N(1) 0.218 06(12) Co(1)-O(2) 0.208 54(11) O(1)_Co(1)-O(1)#1 180.00(7) O(1)-Co(1)-O(2) 88.61(5) O(1)#1-Co(1)-O(2) 91.39(5) O(1)-Co(1)-O(2)#1 91.39(5) O(2)-Co(1)-O(2)#1 180.00(8) O(2)-Co(1)-N(1)#1 91.04(4) O(1)-Co(1)-N(1)#1 87.54(5) O(1)-Co(1)-N(1) 92.46(5) O(1)#1-Co(1)-N(1) 87.54(5) N(1)-Co(1)-O(2) 88.96(4) O(2)#1-Co(1)-N(1) 91.04(4) N(1)#1-Co(1)-N(1) 180.00(16) Compound 2 Co(1)-N(1) 0.216 16(11) Co(1)-O(1) 0.209 37(11) Co(1)-O(2) 0.213 65(11) O(2)-Co(1)-N(1) 89.77(4) O(1)-Co(1)-O(2)#2 93.38(4) O(2)-Co(1)-O(2)#2 180 O(1)#2-Co(1)-O(1) 180 O(1)-Co(1)-O(2) 86.62(4) N(1)#2-Co(1)-N(1) 180.00(3) O(1)-Co(1)-N(1) 87.11(4) O(2)#2-Co(1)-N(1) 90.23(4) O(1)#2-Co(1)-N(1) 92.89(4) Symmetry codes: #1:-x+1, -y+1, -z for 1; #2:-x+1, -y, -z+1 for 2. -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 0
- 文章访问数: 571
- HTML全文浏览量: 67


 下载:
下载:



 下载:
下载:

