
Citation: Shao-shen Xu, Miao Du, Yi-hu Song, Zi-liang Wu, Qiang Zheng. Effect of Sodium Dodecyl Sulfate on the Rheological Behavior of Poly(vinyl alcohol) Aqueous Solution[J]. Acta Polymerica Sinica, 2020, 51(4): 403-410. doi: 10.11777/j.issn1000-3304.2019.19178

十二烷基硫酸钠对聚乙烯醇亚浓水溶液流变行为的影响
English
Effect of Sodium Dodecyl Sulfate on the Rheological Behavior of Poly(vinyl alcohol) Aqueous Solution
-
Key words:
- Poly(vinyl alcohol)
- / Sodium lauryl sulfate
- / Hydrogen bond
- / Rheological behavior
-
聚乙烯醇(polyvinyl alcohol,简称PVA)是一种水溶性极好的乙烯基聚合物,并具有优异的生物相容性和可降解性[1]. 由于其出色的化学和物理性质,PVA被广泛用于工业、商业、农业、医疗等各个领域[2~4],主要用作胶体分散的乳化剂、黏合剂、人造关节凝胶、生物医用材料等[5~8]. PVA多以水溶液状态进行加工与使用,为优化PVA加工工艺及使用性能,必须研究PVA水溶液的流变行为[9,10]. PVA具有较规整的链结构,是一种半结晶性聚合物,侧链上羟基的存在使其分子链内及分子链间易形成氢键[9]. 其中,分子间氢键的形成会大大增加PVA水溶液的黏度[11],给加工过程的顺利进行带来一定困难. 为此,常加入表面活性剂、小分子盐、无机粒子[12~14]等来调控其黏度. 十二烷基硫酸钠(sodium dodecyl sulfate,简称SDS)是一种阴离子表面活性剂,常与一些聚合物水溶液复合使用[15,16]. SDS添加至PVA水溶液中,会与PVA大分子产生相互作用,对其流变行为造成一定影响[17,18]. Ye等研究发现,向10 wt% PVA水溶液中加入SDS(用量为PVA的1 wt%),PVA水溶液的黏度明显下降[19]. 然而,SDS含量变化对于PVA水溶液的流变行为影响的具体机理尚不完全清楚. 另一方面,实际生产中所用的PVA水溶液均为亚浓溶液,本文详细考察了较宽范围内,SDS含量对PVA亚浓水溶液流变行为的影响,分析了SDS对于PVA亚浓水溶液降黏效果的影响,探讨了降黏机理.
1. 实验部分
1.1 实验原料
本文使用的PVA(牌号:1750)聚合度为1750 ± 50,醇解度为98% ~ 99%,分析纯,国药集团化学试剂有限公司产品;表面活性剂十二烷基硫酸钠(SDS,分析纯),国药集团化学试剂有限公司产品,实验过程中所用水均为去离子水.
1.2 PVA/SDS水溶液的配制
本文配制了不同浓度的PVA/SDS复合水溶液,以浓度为10 wt%的PVA水溶液为例,将5 g PVA颗粒和一定量SDS分散于50 mL去离子水中,搅拌速率调至200 r/min,110 °C油浴加热,待PVA颗粒在SDS溶液中完全溶解后,停止加热,自然冷却至室温,将溶液倒入锥形瓶密封保存,用于流变行为及其他性能测试.
1.3 测试方法
1.3.1 流变行为测试
用DHR旋转流变仪(美国TA公司)对PVA溶液的流变行为进行测试,采用直径为40 mm的锥形板夹具,测试亚浓溶液的黏度;采用直径为60 mm的锥形板夹具,测试稀溶液黏度;采用直径为40 mm的平行板夹具,进行动态温度扫描和应变扫描测试. 稳态扫描的剪切速率范围为0.001 ~ 1000 s−1,每一剪切速率(
$\dot \gamma $ 1.3.2 熔融焓测试
用Q25示差扫描量热仪(美国TA公司)测试样品的熔融焓,称取5 ~ 10 mg试样密封于液体坩埚,先降至233 K冷冻后,以5 K/min的速率升温至313 K,防止升温过快导致热效应滞后现象的发生.
1.3.3 接触角的测试
用接触角测量仪测定样品的接触角,以聚四氟乙烯板为基板,利用移液枪移取5 ~ 10 mg试样于聚四氟乙烯平板上,待液滴稳定后,调整焦距以及光强,使图像清晰后读取接触角示数.
2. 结果与讨论
2.1 SDS在PVA亚浓水溶液中临界聚集浓度和临界胶束浓度
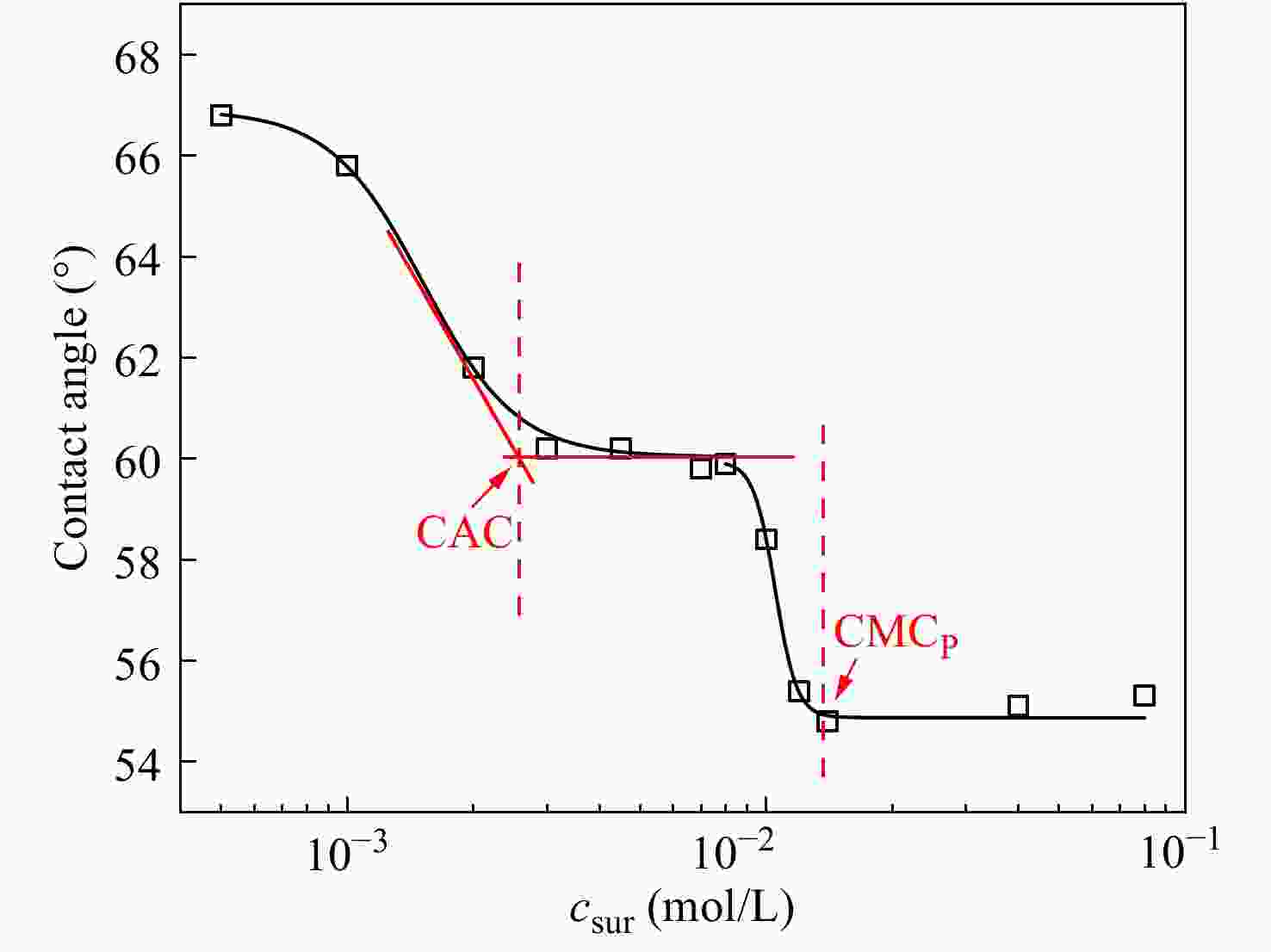
无聚合物的表面活性剂水溶液中,当表面活性剂增加到一定浓度时,活性剂分子会在溶液中聚集形成胶束,该浓度即为临界胶束浓度(critical micelle concentration,CMC). 而在有聚合物存在的表面活性剂溶液中,表面活性剂达到一定浓度(csur)后,会优先与大分子链发生作用,沿大分子链形成类似胶束的聚集体[20],该浓度称为临界聚集浓度(critical aggregation concentration,CAC). 随着csur继续增大,表面活性剂被大分子链的吸附达饱和后,才会在水中发生聚集,形成胶束,该浓度是聚合物溶液中表面活性剂的临界胶束浓度(CMCP). CAC一般比CMCP低1 ~ 2个数量级[21,22]. CAC的大小可用来判断聚合物与表面活性剂之间相互作用的强弱,相互作用越强,CAC越小,反之亦然[23,24].
表面活性剂使液面的表面张力(
$\varGamma $ $\varGamma $ $\varGamma $ ${\varGamma ^{\rm sg}}$ ${\varGamma ^{\rm sl}}$ ${\varGamma ^{\lg }}$ $\varGamma $ $\varGamma $ $\varGamma $ $ \cos \theta = \frac{{{\varGamma ^{\rm sg}} - {\varGamma ^{\rm sl}}}}{{{\varGamma ^{\lg }}}} $ (1) 本文配制了一系列不同csur的PVA/SDS复合溶液(PVA的浓度固定为10 wt%),以聚四氟乙烯板作为基板,测试了不同液滴的θ,并对csur作图,见图1. 可以看出,曲线出现2个拐点,第一个拐点为0.002 mol/L,即为SDS在10 wt% PVA溶液中的CAC值,此时SDS分子开始与PVA主链发生相互作用,被吸附在PVA主链上并聚集,以吸附在聚合物上的胶束形式存在. 随着csur的继续升高,PVA主链全部被SDS胶束所占据,此后SDS开始在水中聚集形成胶束,与之相对应,在0.012 mol/L处出现第二个拐点,即为SDS在10 wt% PVA水溶液中的临界胶束浓度(CMCP). SDS在纯水中的CMC值约为0.008 mol/L. 对比发现,SDS在10 wt% PVA溶液中的CMCP略高于SDS在纯水中的CMC,这是因为在高分子溶液中,csur较低时,SDS分子首先与PVA大分子链发生作用,被吸附于大分子链上并聚集,但并未形成独立的SDS胶束;随着csur增大,大分子链上吸附的SDS趋于饱和,才开始溶于水中,继而形成胶束,故SDS的CMCP值相对较高,但由于其CAC较低,故二者差别不大.
图 1
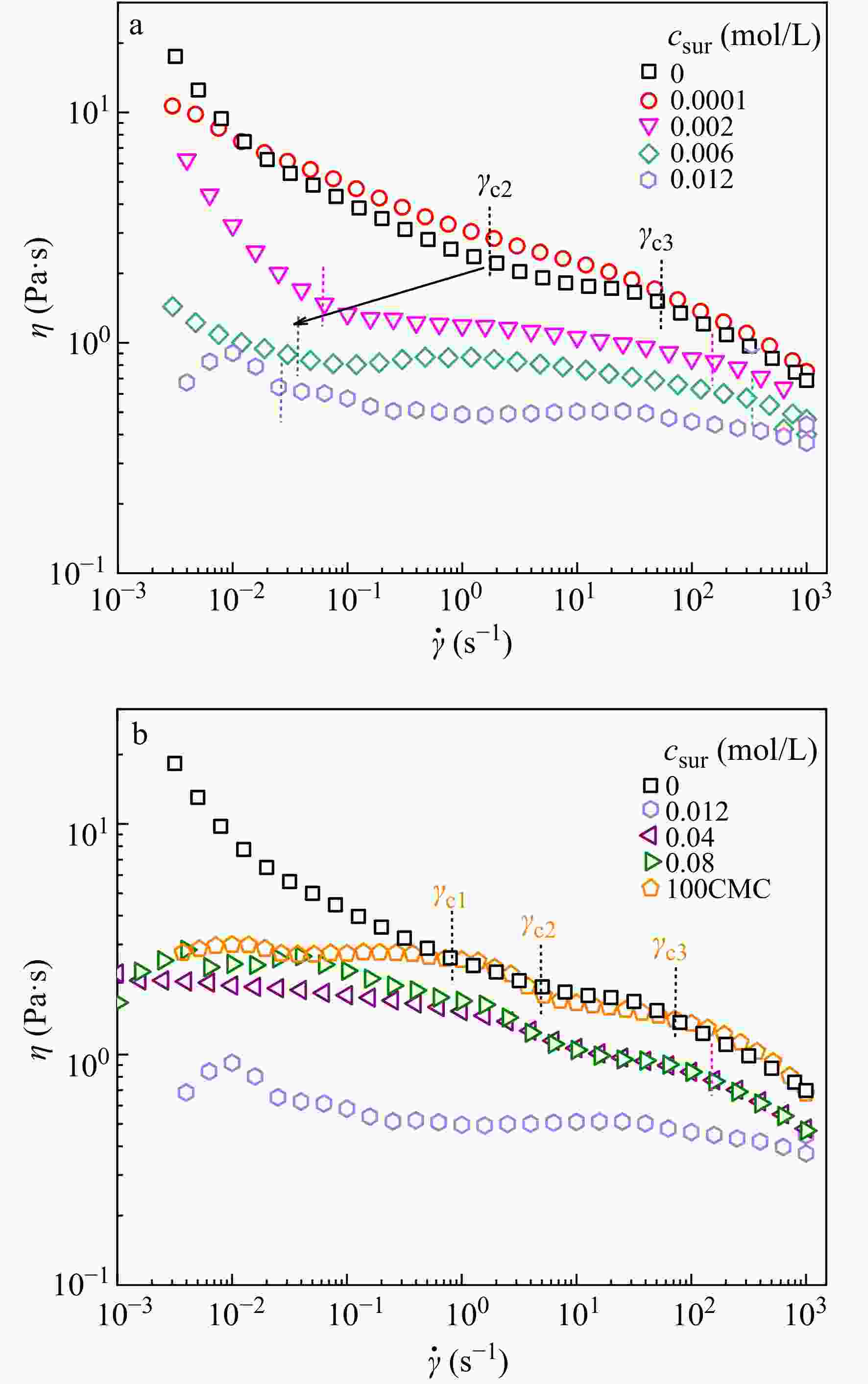
2.2 SDS浓度对PVA亚浓水溶液稳态流变行为的影响
SDS是一种阴离子表面活性剂,其亲水基团―
${\rm{OSO}}_3^{\ 2 - } $ $\dot \gamma $ $\dot \gamma $ ${\dot \gamma _{c1}}$ ${\dot \gamma _{c2}}$ ${\dot \gamma _{c3}}$ ${\dot \gamma _{c2}}$ ${\dot \gamma _{c3}}$ ${\dot \gamma _{c2}}$ ${\dot \gamma _{c3}}$ $\dot \gamma $ $\dot \gamma $ ${\dot \gamma _{c2}}$ $\dot \gamma $ ${\dot \gamma _{c3}}$ $\dot \gamma $ ${\dot \gamma _{c2}}$ $\dot \gamma $ $\dot \gamma $ $\dot \gamma $ ${\dot \gamma _{c3}}$ 图 2
由图2可知,
${\dot \gamma _{c3}}$ ${c}^* $ ${{c}}^* = 3{M_{{\rm n}}}/4{\text{π}} R_{{\rm g}}^{{3}}{N_{\rm A}}$ ${\dot \gamma _{c3}}$ ${\tau _{\rm r}}$ ${\dot \gamma _{c3}}$ ${\tau _{\rm r}}$ ${\tau _{\rm r}} = {\left( {\dfrac{N}{g}} \right)^3}{\tau _{\rm blob}}$ ${\tau _{\rm blob}} = \dfrac{{{\eta _{\rm s}}{\xi ^3}}}{{{k_{\rm{B}}}T}}$ ${\eta _{\rm s}}$ ${\tau _{\rm blob}}$ ${\dot \gamma _{c3}}$ ${\tau _{\rm r}}$ 在
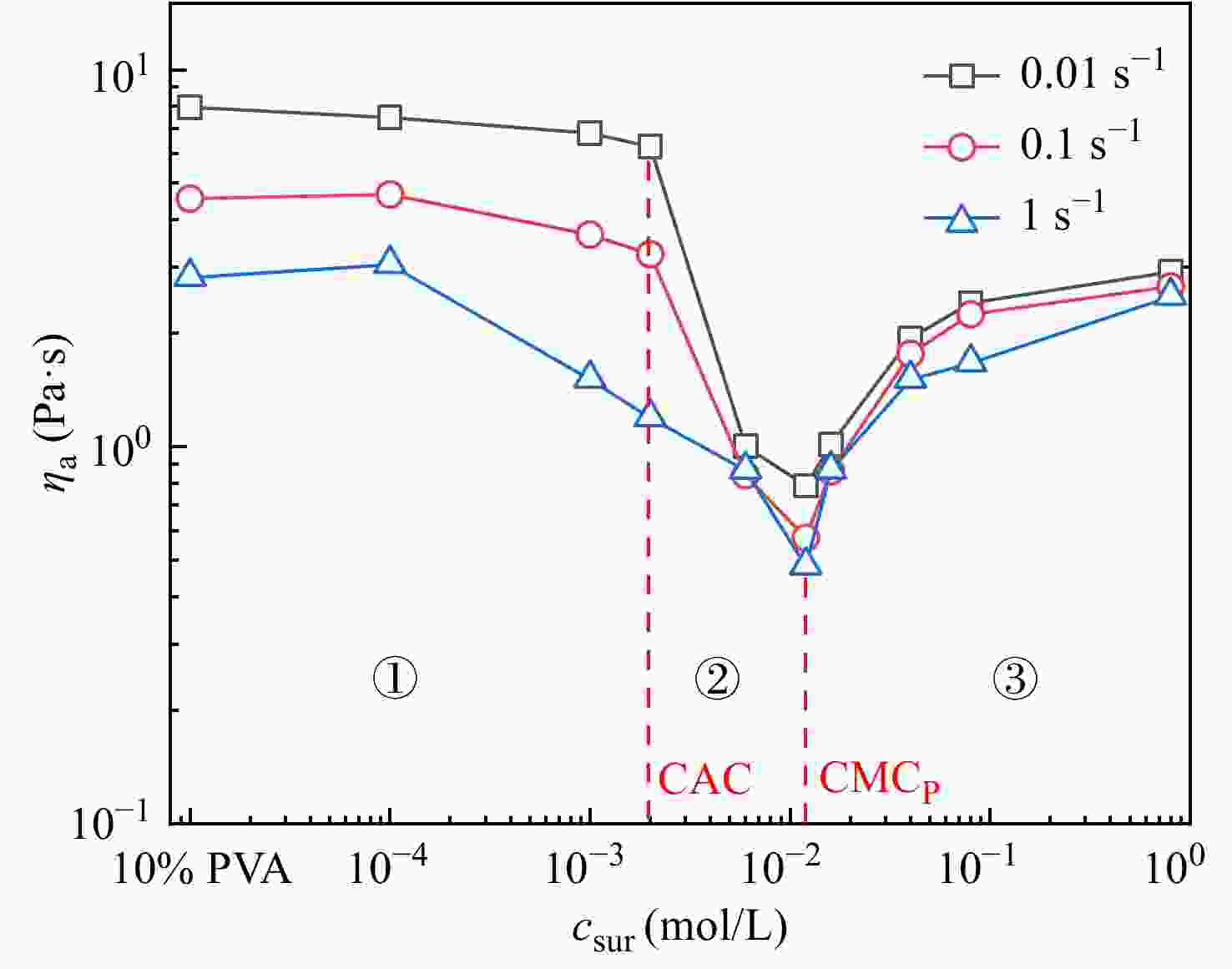
${\dot \gamma _{c2}}$ $\dot \gamma $ ${\dot \gamma _{c3}}$ ${\eta _{\rm a}}$ ${\dot \gamma _{c2}}$ $\dot \gamma $ ${\dot \gamma _{c2}}$ ${\dot \gamma _{c3}}$ $\dot \gamma $ ${\eta _{\rm a}}$ $\dot \gamma $ ${\eta _{\rm a}}$ ${\eta _{\rm a}}$ ${\eta _a}$ ${\eta _{\rm a}}$ ${\eta _{\rm a}}$ ${\eta _{\rm a}}$ ${\eta _a}$ ${\eta _{\rm a}}$ 图 3
PVA分子链具有较规整的结构,侧链上的羟基使其水溶液体系中存在较多分子内、分子间和PVA与水分子间等3种氢键相互作用. 正是由于氢键相互作用的存在,使PVA溶液具有较大的黏度. 而SDS是一种阴离子表面活性剂,SDS带有―
${\rm{OSO}}_3^{2 - } $ ${\eta _{\rm a}}$ 从图3还可以看到,第一区(低csur区),低
$\dot \gamma $ ${\eta _{\rm a}}$ $\dot \gamma $ ${\eta _{\rm a}}$ $\dot \gamma $ ${\eta _{\rm a}}$ ${\eta _{\rm a}}$ 2.3 SDS对PVA水溶液水合数的影响
根据稳态流变测试结果,SDS对PVA水溶液具有一定降黏效果,最大可使PVA溶液
${\eta _{\rm a}}$ 图 4
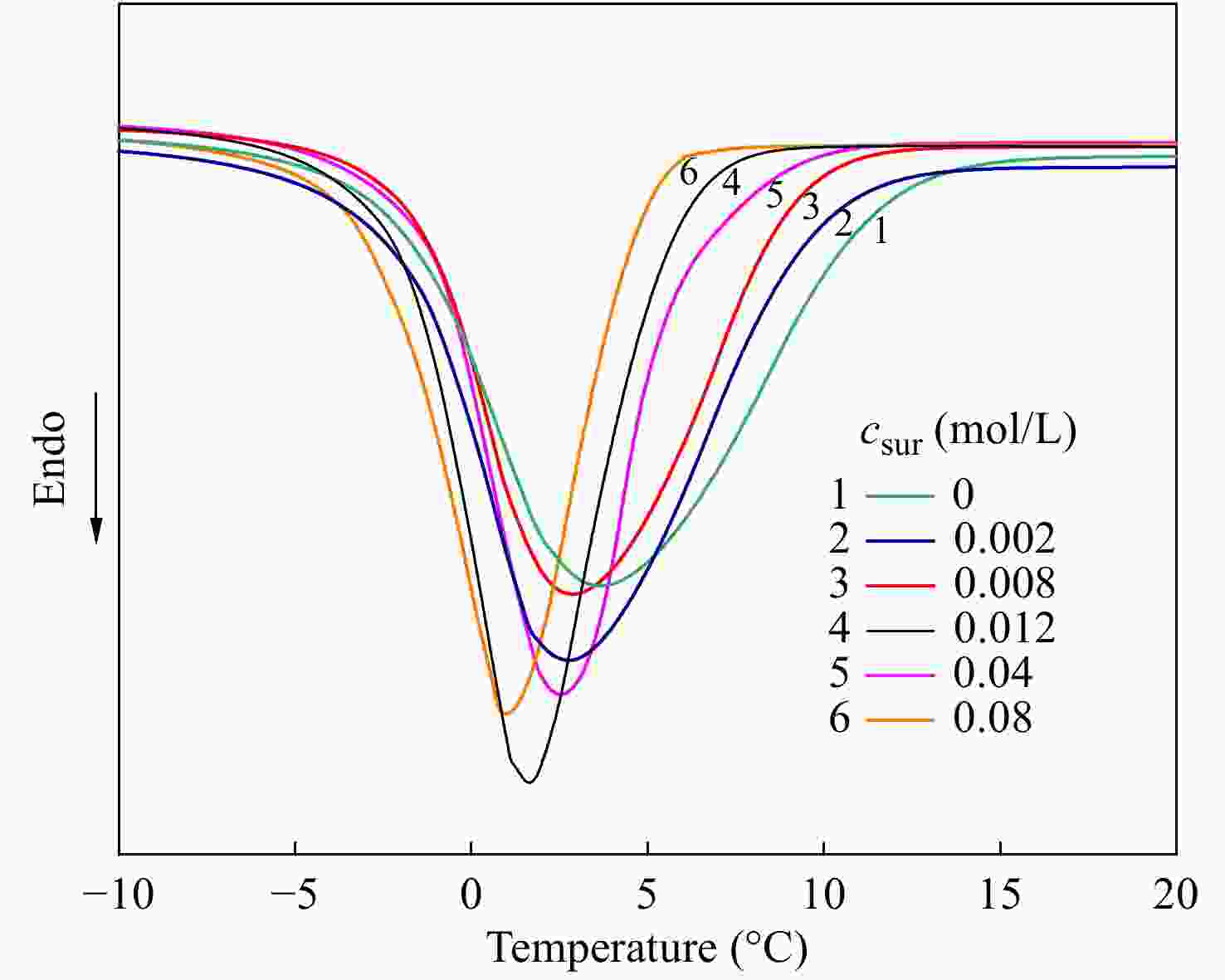
水分子在聚合物的水溶液中以结合水和自由水2种状态存在,用水合数(n)来定量描述每个PVA重复单元的结合水的数量,如下式计算[31],
$ n = \left[\left(1 - \frac{{{{\Delta }}{H_{\rm{m}}}}}{{{{\Delta }}H}}\right)\frac{1}{c} - 1 \right]\frac{{{M_0}}}{{{M_{{{\rm{H}}_{\rm{2}}}{\rm{O}}}}}} $ (2) 其中ΔHm和ΔH分别是测试溶液和水的熔融焓,c为PVA溶液的浓度(10 wt%),M0和
${\rm M}_{\rm H_2 O} $ 表 1
csur (mol/L) 0 0.002 0.008 0.012 0.04 0.08 n 2.63 2.61 2.51 2.46 2.43 2.43 本文均使用10 wt%的PVA水溶液,浓度相同,说明体系中―OH的量是一定的,本身形成的氢键网络和结合水的数目均相同. 随着SDS的加入,n由2.63降至2.43,说明SDS基团与PVA链上的羟基发生了相互作用,降低了PVA链上―OH与水的结合数目. 而当csur > CMCP后,n值几乎不变,也就是说,大量SDS的加入后主要形成了胶束,而未被PVA分子链吸附.
2.4 温度对10 wt% PVA/SDS水溶液流变行为的影响
黏流活化能(Ea)可以反映体系黏度对温度(T)的依赖性,即流动过程中,流动单元从原位克服周围分子作用跃迁到附近空穴所需的最小能量. 分子间作用力越大,Ea越高. 一般认为,体系中含有较多氢键相互作用时,Ea会增大,导致其对温度的变化更加敏感. 对于PVA/SDS溶液体系,氢键网络的破坏也可通过Ea的变化间接反映.
${\eta _{\rm a}}$ $ {\eta _{\rm a}} = A\exp \left(\frac{{{E_{\rm a}}}}{{RT}}\right) $ (3) 式中A是前置因子,Ea是黏流活化能,R是理想气体常数,对上式取对数得:
$ \ln {\eta _{\rm{a}}} = \frac{{{E_{\rm a}}}}{R} \cdot \frac{1}{T} + \ln A $ (4) 本文分别取10 wt% PVA和csur分别为0.002、0.006、0.012和0.04 mol/L的复合溶液,即SDS浓度分别为CAC、CAC与CMC之间、CMCP及CMCP以上4个浓度,在
$\dot \gamma $ ${\eta _{\rm a}}$ ${\eta _{\rm a}}$ ${\eta _{\rm a}}$ ${\eta _{\rm a}}$ 表 2
csur (mol/L) 0 0.002 0.006 0.012 0.04 Ea (kJ/mol) 37.22 36.89 30.13 20.93 31.38 图 5
2.5 SDS浓度变化对10 wt%PVA水溶液动态流变行为的影响
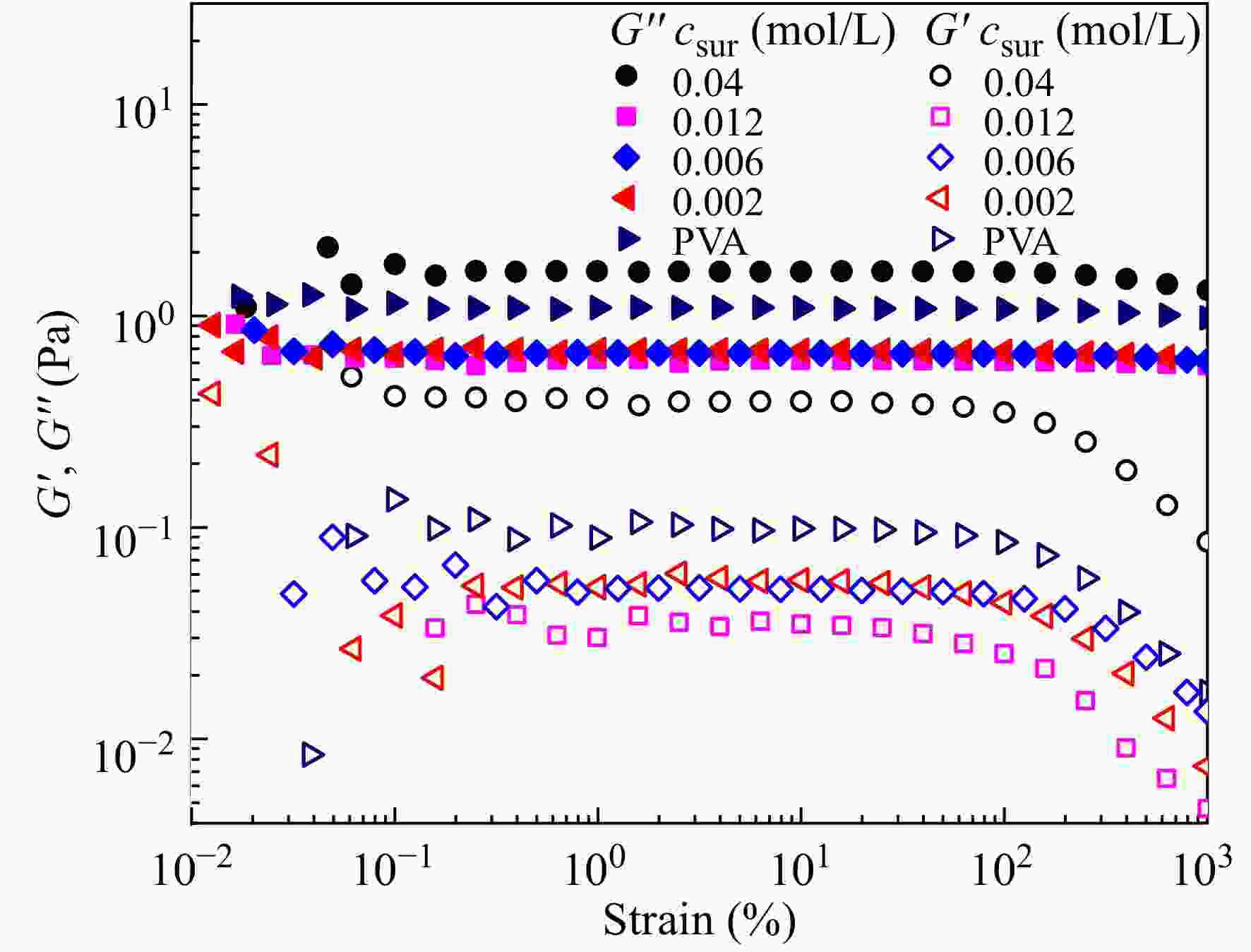
为验证SDS对PVA溶液优异的降黏行为,图6给出了ω = 1 rad/s时,PVA/SDS复合水溶液的应变扫描曲线. 在整个应变测试范围内,G″ > G′说明溶液在该频率下以黏性为主. 当csur = CAC时,溶液的G′与G″均开始逐渐下降,当csur = CMCP时,体系的G′与G″均降至最低,值得注意的是此时G″下降幅度不大,而G′出现了较明显的下降,说明SDS的加入主要引起体系储能模量下降. 当csur = 0.04 mol/L时,G′和G″均发生了较大的增加,而G′的增加幅度大于G″的增加幅度,表明SDS在水中形成的胶束对溶液弹性贡献更大,另外,该体系线性黏弹区较其它体系稍有变窄,源于胶束使体系内形成的物理交联网络结构.
图 6
2.6 SDS对不同浓度PVA流变行为的影响
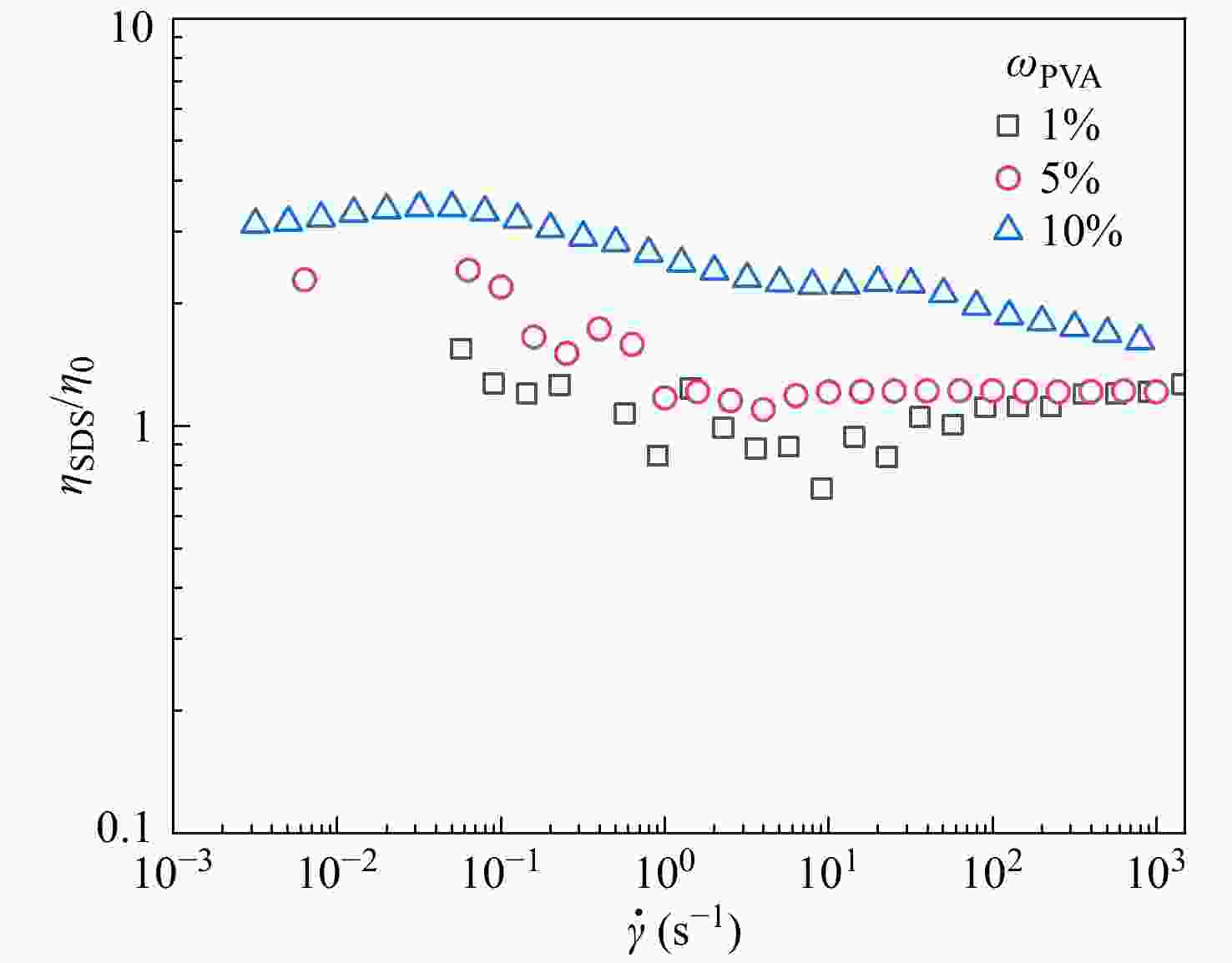
为探究SDS对于不同浓度PVA溶液的降黏效果,本文分别向10 wt%、5 wt%、1 wt% PVA的水溶液加入0.012 mol/L (CMCP值)的SDS进行测试. 为了清晰地表达SDS对PVA溶液的降黏效果,用
${\eta _{{\rm{SDS}}}}$ ${\eta _0}$ ${\eta _{{\rm{SDS}}}}/{\eta _0}$ ${\eta _{{\rm{SDS}}}}/{\eta _0}$ $\dot \gamma $ 图 7
3. 结论
不同浓度SDS对PVA水溶液稳态流变行为的影响可分为3个区域,① 当csur < CAC时,随着csur增加,复合溶液的
${\eta _{\rm a}}$ ${\eta _{\rm a}}$ ${\eta _{\rm a}}$ ${\eta _{\rm a}}$ ${\dot \gamma _{c2}}$ ${\dot \gamma _{c3}}$
-
-
[1]
Chiellini E, Corti A, Dantone S. Prog Polym Sci, 2003, 28(6): 963 − 1014 doi: 10.1016/S0079-6700(02)00149-1
-
[2]
Demerlis C C, Schoneker D R. Food Chem Toxicol, 2003, 41(3): 319 − 326 doi: 10.1016/S0278-6915(02)00258-2
-
[3]
Krumova M, Lopez D, Benavente R. Polymer, 2000, 41(26): 9265 − 9272 doi: 10.1016/S0032-3861(00)00287-1
-
[4]
Ding Wei(丁维), Du Miao(杜淼), Zheng Qiang(郑强). Journal of Zhejiang University (Engineering Science)(浙江大学学报(自然科学版)), 2013, 47(6): 1109 − 1113
-
[5]
Stammen J A, Williams S, Ku D N. Biomaterials, 2001, 22(8): 799 − 806 doi: 10.1016/S0142-9612(00)00242-8
-
[6]
Chen K C, Lin Y F. Enzyme Microb Tech, 1994, 16(1): 79 − 83 doi: 10.1016/0141-0229(94)90113-9
-
[7]
Zheng H, Du Y M, Yu J H. J Appl Polym Sci, 2001, 80(13): 2558 − 2565 doi: 10.1002/app.1365
-
[8]
Gao Hanwen(高瀚文), He Jiyu(何吉宇), Yang Rongjie(杨荣杰), Yang Lei(杨磊). Polymer Materials Science and Enguneering(高分子材料科学与工程), 2010, 26: 65 − 67
-
[9]
Mei Qingqing(梅清青), Du Miao(杜淼). Concentration and time dependence of rheological behavior of polyvinylalcohol aqueous solution(聚乙烯醇水溶液流变行为的浓度及时间依赖性). In: Chinese Society of Rheology, ed.Proceedings of the 13th National Conference on Rheology. Xian: Chinese Society of Rheology, 2016. 123 − 126
-
[10]
Briscoe B J, Luckham P F, Zhu S. Polymer, 2000, 41: 3851 − 3860 doi: 10.1016/S0032-3861(99)00550-9
-
[11]
Satokawa Y, Shikata T. Macromolecules, 2008, 41: 2908 − 2913 doi: 10.1021/ma702793t
-
[12]
Zhao J H, Xu A H, Yuan W Z, Gao J B, Tang J K, Wang L, Ai F, Zhang Y M. J Mater Sci, 2011, 46: 7501 − 7510 doi: 10.1007/s10853-011-5721-3
-
[13]
Son W K, Youk J H, Lee T S, Park W H. Mater Lett, 2005, 59(12): 1571 − 1575 doi: 10.1016/j.matlet.2005.01.025
-
[14]
Lv Weiyang(吕维扬), Ye Weijuan(叶维娟), Fu Huakang(傅华康), Du Miao(杜淼), Zheng Qiang(郑强). Acta Polymerica Sinica(高分子学报), 2015, (6): 699 − 705
-
[15]
Huang Chuanjing(黄传敬). Journal of Huaibei Normal University(淮北煤师院学报), 1997, (1): 45 − 48
-
[16]
Zhang Qun(张群), Pei Meishan(裴梅山), Zhang Jin(张瑾), Li Wenwei(李文薇), Li Cheng(李诚). China Surfactant Detergent & Cosmetics(日用化学工业), 2006, (2): 69 − 72 doi: 10.3969/j.issn.1001-1803.2006.02.001
-
[17]
Weng Jianxin(翁建新), Hong Zhangzhu(洪掌珠). Journal of Yunnan Normal University(云南师范大学学报), 2005, 25: 58 − 61
-
[18]
Cheng R S, Yang Y, Yan X H. Polymer, 1999, 40(13): 3773 − 3779 doi: 10.1016/S0032-3861(98)00608-9
-
[19]
Ye Weijuan(叶维娟), Lv Weiyang(吕维扬), Mei Qingqing(梅清青), Fu Huakang(傅华康), Du Miao(杜淼),Zheng Qiang(郑强). Acta Polymerica Sinica(高分子学报), 2015, (10): 1216 − 1222 doi: 10.11777/j.issn1000-3304.2015.15073
-
[20]
Holmberg K, Jonsson B, Kronberg B, Lindman B. Surfactants and Polymers in Aqueous Solution. West Sussex, England: John Wiley & Sons Ltd, 2002. 278 − 284
-
[21]
Plucktaveesak N, Konop A J, Colby R H. J Phys Chem B, 2003, 107(32): 8166 − 8171 doi: 10.1021/jp0275995
-
[22]
Merta J, Stenius P. Colloid Surf A - Physicochem Eng Asp, 1997, 122(1-3): 243 − 255 doi: 10.1016/S0927-7757(96)03823-X
-
[23]
Hansson P, Almgren M. Langmuir, 1994, 10(7): 2115 − 2124 doi: 10.1021/la00019a017
-
[24]
Piculell L, Lindman B. Adv Colloid Interface, 1992, 41: 149 − 178 doi: 10.1016/0001-8686(92)80011-L
-
[25]
Wu Q, Du M, Shangguan Y G, Zhou J P, Zheng Q. Colloid Surf A-Physicochem Eng Asp, 2009, 332: 13 − 18 doi: 10.1016/j.colsurfa.2008.08.022
-
[26]
Zhao Guoxi(赵国玺). Principles of Surfactant Action(表面活性剂的作用原理). Beijing(北京): China Light Industry Press(中国轻工业出版社), 2003. 416 − 436
-
[27]
Wu Q, Du M, Ye T, Shangguan Y G, Zhou J P, Zheng Q. Colloid Polym Sci, 2009, 287: 911 − 918 doi: 10.1007/s00396-009-2045-9
-
[28]
de Gennes P G. Scaling Concepts in Polymer Physics. New York: Cornell University Press, 1979. 228 ~ 229
-
[29]
Doi M, Edwards S F. J Chem Soc Faraday Trans II, 1979, 75: 38 − 54 doi: 10.1039/F29797500038
-
[30]
Zhao Pingying(赵平英), Yang Zhenyu(杨震宇), Gao Lijun(高立军). A rough estimate of surfactant SDS micelle size parameters(表面活性剂SDS胶束尺寸参数的粗略估算). In: Chinese Chemical Society, ed. 13th National Conference on Chemical Thermodynamics and Thermal Analysis Abstract Set of Papers. Xinxiang: Chinese Chemical Society, 2006. 227
-
[31]
Li W B, Zheng Y, Cheng R S. Polymer, 2008, 29: 4740 − 4744
-
[1]
-
Table 1. Hydration numbers of PVA solution with various SDS concentrations
csur (mol/L) 0 0.002 0.008 0.012 0.04 0.08 n 2.63 2.61 2.51 2.46 2.43 2.43 Table 2. Viscous activation energy of PVA solution with different SDS concentrations
csur (mol/L) 0 0.002 0.006 0.012 0.04 Ea (kJ/mol) 37.22 36.89 30.13 20.93 31.38 -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 97
- 文章访问数: 8181
- HTML全文浏览量: 1540

 下载:
下载:

 下载:
下载:

