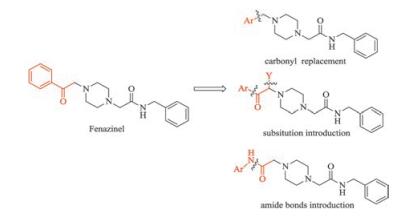
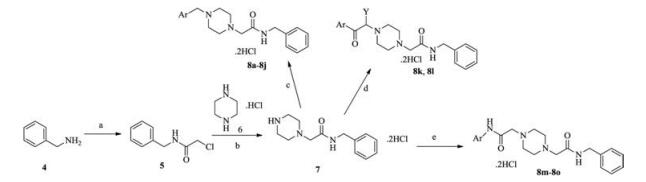
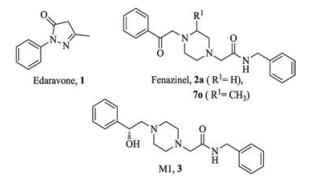
-
[1]
Towfighi A., Ovbiagele B., Saver J.L.. Therapeutic milestone: stroke declines from the second to the third leading organ-and disease-specific cause of death in the United States[J]. Stroke,
2010,41:499-503.
doi: 10.1161/STROKEAHA.109.571828
-
[2]
Fang X.H., Kronmal R.A., Li S.C.. Prevention of stroke in urban China: a community-based intervention trial[J]. Stroke,
1999,30:495-501.
doi: 10.1161/01.STR.30.3.495
-
[3]
Fang M.C., Go A.S., Chang Y.. Long-term survival after ischemic stroke in patients with atrial fibrillation[J]. Neurology,
2014,82:1033-1037.
doi: 10.1212/WNL.0000000000000248
-
[4]
Hsu C.Y.. Ischemic Stroke: from Basic Mechanisms to New Drug Development[J]. Karger Basel,
1998.
-
[5]
Nicole O., Docagne F., Ali C.. The proteolytic activity of tissueplasminogen activator enhances NMDA receptor-mediated signaling[J]. Nat. Med.,
2001,7:59-64.
doi: 10.1038/83358
-
[6]
Molina C.A., Montaner J., Abilleira S.. Time course of tissue plasminogen activator-induced recanalization in acute cardioembolic stroke: a case-control study[J]. Stroke,
2001,32:2821-2827.
doi: 10.1161/hs1201.99821
-
[7]
Menon B.K., Saver J.L., Prabhakaran S.. Risk score for intracranial hemorrhage in patients with acute ischemic stroke treated with intravenous tissue-type plasminogen activator[J]. Stroke,
2012,43:2293-2299.
doi: 10.1161/STROKEAHA.112.660415
-
[8]
Cucchiara B., Tanne D., Levine S.R., Demchuk A.M., Kasner S.. A risk scoretopredict intracranial hemorrhage after recombinant tissue plasminogen activator for acute ischemic stroke[J]. J. Stroke Cerebrovasc. Dis.,
2008,17:331-333.
doi: 10.1016/j.jstrokecerebrovasdis.2008.03.012
-
[9]
Whiteley W.N., Thompson D., Murray G.. Targeting recombinant tissuetype plasminogen activator in acute ischemic stroke based on risk of intracranial hemorrhage or poor functional outcome: an analysis of the third international stroke trial[J]. Stroke,
2014,45:1000-1006.
doi: 10.1161/STROKEAHA.113.004362
-
[10]
Feng S., Yang Q., Liu M.. Edaravone for acute ischaemic stroke[J]. Cochrane Database Syst Rev.,
2011,12CD007230.
-
[11]
Kitagawa Y.. Edaravone in acute ischemic stroke[J]. Int. Med.,
2006,45:225-226.
doi: 10.2169/internalmedicine.45.0143
-
[12]
Kikuchi K., Miura N., Kawahara K.. Edaravone (Radicut) a free radical scavenger, is a potentially useful addition to thrombolytic therapy in patients with acute ischemic stroke[J]. Biomed. Rep.,
2013,1:7-12.
-
[13]
Wada T., Yasunaga H., Inokuchi R.. Effects of edaravone on early outcomes in acute ischemic stroke patients treated with recombinant tissue plasminogen activator[J]. J. Neurol. Sci.,
2014,345:106-111.
doi: 10.1016/j.jns.2014.07.018
-
[14]
Takenaka K., Kato M., Yamauti K., Hayashi K.. Simultaneous administration of recombinant tissue plasminogen activator and edaravone in acute cerebral ischemic stroke patients[J]. J. Stroke Cerebrovasc. Dis.,
2014,23:2748-2752.
doi: 10.1016/j.jstrokecerebrovasdis.2014.06.016
-
[15]
Patel D.A.. A review on pharmacology of combined edaravone and argatroban therapy in acute ischemic stroke[J]. Pharmatutor,
2014,2:42-49.
-
[16]
J. Q. Li, L. Y. Huang, Y. Min, Z. J. Weng, C. N. Zhang, Aralkyl formyl-alkyl piperazine derivatives and their uses as a cerebral nerve protective agent, US 7326710.
-
[17]
Li J.Q., Huang L.Y., Xia Y.Y.. Synthesis of aroylpiperazine derivatives and their anti-cerebral anoxia, anti-cerebral ischemia biological activities[J]. Chin. J. Med. Chem.,
2006,16:6-14.
-
[18]
Zhao T., Wei Z., Shen F.M.. Protective effects of fenazinel dihydrochloride against stroke in stroke-prone spontaneously hypertensive rats[J]. Acad. J. Second Mil. Med. Univ.,
2011,32:1282-1285.
-
[19]
Li D.J., Li J.Q., Huang L.Y.. Protective effects of fenazinel dihydrochloride on focal cerebral ischemic injury in rats[J]. Chin. Pharmacol. Bull.,
2009,25:716-720.
-
[20]
Jin L.L., Sheng Y.C., Zhong Y., Zhu P., Xia Y.Y.. Relation between therapeutic effects and administration time of fenazinel dihydrochloride on focal cerebral ischemia injury in rats[J]. Chin. J. Pharm.,
2008,39:356-358.
-
[21]
Chen Y.F., Min L., Zhang B., Xie B.Y.. Preparation of fenazinel dihydrochloride injection[J]. Chin. J. Pharm.,
2007,38:852-854.
-
[22]
Huang L.Y., Weng Z.J., Huang L., Li J.Q.. Synthesis of fenazinel dihydrochloride[J]. Chin. J. Pharm.,
2007,38:191-193.
-
[23]
Wang W.Y., Shen C.W., Weng Z.J.. synthesis and biological evaluation of novel dicarbonylalkyl piperazine derivatives as neuroprotective agents[J]. Chin. Chem. Lett.,
2016,27:387-390.
doi: 10.1016/j.cclet.2015.11.002
-
[24]
Recanatini M., Poluzzi E., Masetti M., Cavalli A., De Ponti F.D.. QT prolongation through hERG K+ channel blockade: Current knowledge and strategies for the early prediction during drug development[J]. Med. Res. Rev.,
2005,25:133-166.
doi: 10.1002/(ISSN)1098-1128
-
[25]
Dubin A.E., Nasser N., Rohrbacher J.. Identifying modulators of hERG channel activity using the PatchXpress® planar patch clamp[J]. J. Biomol. Screen.,
2005,10:168-181.
doi: 10.1177/1087057104272295

 Login In
Login In






 DownLoad:
DownLoad: