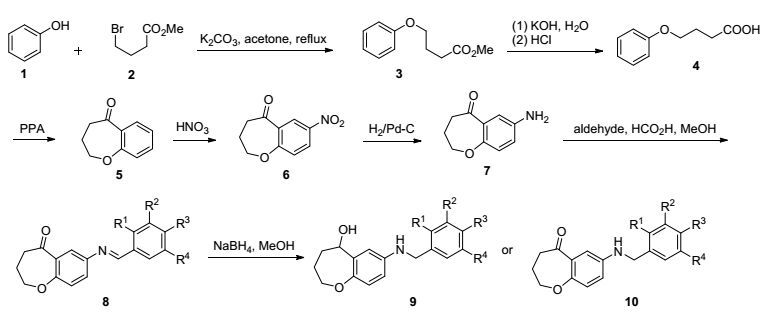
 图 1
化合物8~10合成
Figure Figure1.
Synthesis of compounds 8~10
图 1
化合物8~10合成
Figure Figure1.
Synthesis of compounds 8~10

Citation: Hou Guige, Jiang Chengshi, Liu Hongchun, Tong Linjiang, Peng Xia, Ji Yinchun, Geng Meiyu, Xiao Wei, Gong Jingxu, Guo Yuewei. Synthesis and Activity Evaluation of Novel 3, 4-Dihydro-benzo[b]-oxazepin-5(2H)-one Derivatives as Protein Kinases Inhibitors[J]. Chinese Journal of Organic Chemistry, 2017, 37(6): 1463-1472. doi: 10.6023/cjoc201611005

新颖3,4-二氢-苯并[b]氧杂(艹卓)-5(2H)-酮类化合物的合成以及蛋白酪氨酸激酶抑制活性研究
-
关键词:
- 3,4-二氢-苯并[b]氧杂(艹卓)-5(2H)-酮
- / 合成
- / 蛋白酪氨酸激酶抑制剂
- / ErbB1
- / ErbB2
English
Synthesis and Activity Evaluation of Novel 3, 4-Dihydro-benzo[b]-oxazepin-5(2H)-one Derivatives as Protein Kinases Inhibitors
-
蛋白酪氨酸激酶(Protein-tyrosine kinases, PTKs)是一类具有酪氨酸激酶活性的蛋白质, 它们能催化三磷酸腺苷(ATP)上的磷酸基转移到许多重要蛋白质的酪氨酸残基上, 使其发生磷酸化[1~4].按PTKs结构可以分为受体酪氨酸激酶(RPTK)和非受体酪氨酸激酶(NRPTK). PTKs功能的失调, 会导致其下游信号通路的激活, 进而引起细胞增殖调节紊乱, 最终导致肿瘤形成[5]. RPTK是目前效果明显且前景广阔的抗肿瘤药物靶点之一[6].自2001年起, 美国食品药品监督管理局先后批准了gefitinib, imatinib, erlotinib等10多个小分子蛋白酪氨酸激酶抑制剂上市, 有超过100个正在临床试验阶段的候选药物[7].但这些抑制剂大多存在选择性低或易产生耐药性等问题.因此, 寻找高活性和高选择性的PTK抑制剂仍是肿瘤化学治疗研究的一个重要课题.
由于具有众多的结构特性和生物活性, 天然产物是药物研究中重要的先导化合物来源[8].苯并氧杂䓬酮类化合物经常从天然产物中分离得到, 3, 4-二氢-苯并[b]氧杂䓬-5(2H)-酮类化合物是苯并氧杂䓬酮类化合物的典型代表, 结构中包含了平面的苯环和氧杂䓬-5(2H)-酮药效团. Buckle等[9]发现3, 4-二氢-苯并[b]氧杂䓬-5(2H)-酮类化合物显示了与克罗卡林相似的钾通道激动活性, 与之相似的苯并氧氮杂䓬酮化合物, 药效团上增加了氮原子, 通过抑制Tankyrase对肿瘤细胞(Hep-3B、Hela、MDA-MB-231) 具有显著的抗肿瘤活性[10].另有研究显示此类化合物具有抗炎、抗肿瘤、镇痛等多种生物活性[11~13].近来研究表明此类化合物还具有显著的PTK抑制活性[14], 但迄今为止, 有关此类化合物PTK抑制活性仅有少量的报道.基于寻找具有更好PTK抑制活性的苯并氧杂䓬酮类化合物, 本论文通过系列合成反应在3, 4-二氢-苯并[b]氧杂䓬-5(2H)-酮的苯环上引入含氮基团, 得到一系列的含氮基团取代的3, 4-二氢-苯并[b]氧杂䓬-5(2H)-酮类化合物, 对此类化合物的PTK抑制活性进行了测定, 并初步分析了该类化合物对PTK抑制活性的构效关系.
1 结果与讨论
1.1 化学部分
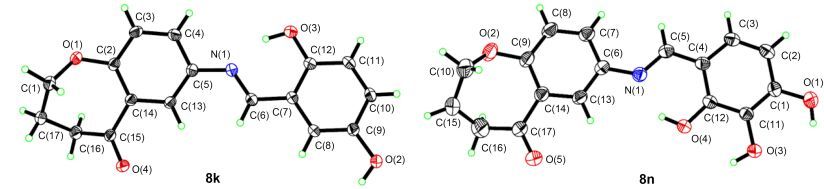
图 1列出了3, 4-二氢-苯并[b]氧杂䓬-5(2H)-酮的合成路线, 参照文献方法[9, 15], 在碳酸钾的丙酮溶液中苯酚与4-溴丁酸反应得到醚化产物3, 经水解得到苯氧基丁酸4, 然后在多聚磷酸(PPA)中加热发生Friedel-Crafts酰基化得到3, 4-二氢-苯并[b]氧杂䓬-5(2H)-酮(5), 将5经发烟硝酸硝化得到7-硝基取代的化合物6, 再经Pd/C催化氢化生成氨基取代的化合物7.在7的基础上, 与不同的醛发生Schiff-base缩合得到不同取代基取代的亚胺衍生物8, 亚胺8经NaBH4还原, 得到醇9或酮10, 合成的化合物如表 1所示.化合物3~7谱图数据与文献一致[16].化合物8~10的结构经NMR和HR-MS的确证, 均为新化合物.化合物8为亚胺类化合物, 存在双键顺反构型, 为进一步验证其构型, 本论文选取8k和8n进行了X单晶衍射分析.如图 2所示, 相对于中心C=N基团, 化合物8k和8n的两个芳环采取反式构型(图 2).在用NaBH4还原亚胺8时, 大部分化合物羰基以及亚胺还原生成醇和胺, 当还原苯环上含有多羟基的化合物时, 仅在亚胺基团发生还原, 而羰基不发生反应.
1.2 生物部分
为研究新化合物的蛋白酪氨酸激酶抑制活性, 本研究采用酶联免疫分析方法(ELISAs)进行评估, 测定了化合物对表皮生长因子受体ErbB1和ErbB2、酪氨酸蛋白激酶受体c-Met、间变性淋巴瘤激酶ALK、成纤维细胞生长因子受体FGFR1以及血管内皮生长因子受体KDR的抑制活性, 其中化合物8h、8i、8m、8n、10a、10b表现出较好的抑制活性, 其余化合物对PTKs的各个因子的抑制率低于50%, 对于测试它们的IC50值没有意义, 因此, 本论文只将部分化合物的IC50值列在表 2中.
如表 2所示, 化合物8i和10a对于大多PTKs具有一定的抑制活性, 其中以8i对ErbB2的活性最强, 其IC50为0.33 μmol/L.另外8h、8i、8n和10b对部分PTKs具有一定的活性, 其中以8n对RET的抑制活性最强, IC50为0.7 μmol/L, 化合物10b对ErbB2的IC50为1.0 μmol/L.虽然活性都不如阳性对照药Lapatinib、PF-2341066、AZD4547和Su11248, 但通过初步的构效关系分析, 为此类化合物的深入研究提供了依据.测试结果显示5-位为羟基结构的化合物9都没有活性, 显示3-位羰基对于活性有重要影响.对比5-位为羰基结构的化合物8和10, 苯环上的取代基对于活性具有重要影响, 当取代基为卤素、甲氧基、硝基等取代基时均无显著活性, 当取代基为多羟基结构时, 化合物呈现较好的活性.在具有多羟基苯酚结构的化合物中, 其中8h、8i、8n、10a和10b等具有邻苯二酚结构片段的化合物具有良好的抑制活性, 而8j、10c等具有间苯二酚结构的化合物皆无活性.
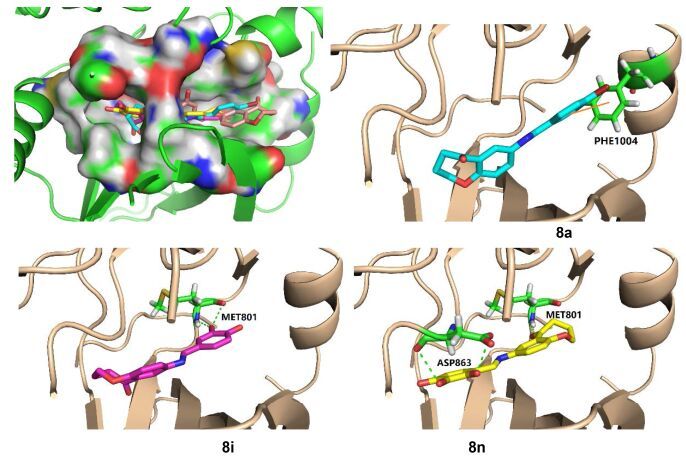
1.3 分子对接研究
我们挑选活性较好的8i、8n以及无活性的8a与蛋白酪氨酸激酶ErbB2蛋白晶体(pdb 3RCD)在Glide 5.5程序上进行分子对接, 以此为例来讨论活性化合物与蛋白酪氨酸激酶的结合过程中酚羟基所起的作用.如图 3所示, 预测的对接模型表明, 化合物8a、8i以及8n作用于ErbB2活性口袋中的空间位置相同:其中, 化合物8i和8n的酚羟基可分别与活性位点的氨基酸Met801和Asp863存在氢键作用, 此外化合物8n的羰基也可与Met801形成氢键作用; 而化合物8a仅与活性位点Phe1004形成π-π作用, 却无明显的氢键作用.结合上述活性结果, 我们认为由于苯环上羟基与活性位点残基形成的氢键作用, 是化合物对蛋白酪氨酸激酶具有抑制活性的关键因素.
2 实验部分
2.1 仪器与试剂
核磁共振由Bruker AMX 400, 500或VarianEM-360, EM-390型核磁共振仪测定, 化学位移以氘代试剂中残留的质子信号为内标; 质谱由Finnigan MAT-95型质谱仪(EIMS、HREIMS)测定.试剂纯化参照文献[16].快速柱层析采用青岛海洋化工厂或烟台化工研究所实验厂生产柱硅胶H (100~200, 200~300目, 10~40 µmo/L), 或者薄层层析硅胶GF254.
2.2 实验方法
2.2.1 化合物8的合成
化合物7 (53 mg, 0.3 mmol)与不同取代的芳香醛(0.3 mmol)溶解于甲醇中(8 mL), 加入一滴甲酸, 反应物于室温搅拌3 h, 薄层色谱(TLC)跟踪反应进程, 反应结束后, 混合物过滤, 滤饼用水洗涤, 甲醇重结晶得到化合物8.
E-7-[(4-溴苯基)甲亚基]氨基-3, 4-二氢-苯并[b]氧杂䓬-5(2H)-酮(8a):收率78%, 浅黄色固体. m.p. 86~87 ℃; 1H NMR (400 MHz, CDCl3) δ: 2.19~2.31 (m, 2H), 2.95 (t, J=6.9 Hz, 2H, ), 4.28 (t, J=6.6 Hz, 2H), 7.13 (d, J=8.6 Hz, 1H), 7.40 (dd, J=8.6, 2.6 Hz, 1H), 7.63 (d, J=8.4 Hz, 2H), 7.65 (d, J=2.6 Hz, 1H), 7.78 (d, J=8.4 Hz, 2H, ), 8.47 (s, 1H); 13C NMR (125 MHz, CDCl3) δ: 26.3, 40.6, 73.0, 120.0, 121.8, 126.0, 128.0, 129.5, 130.2, 132.1, 135.0, 146.5, 158.71, 160.6, 200.5; HRMS (ESI) calcd for C17H15NO2Br[M+H]+ 344.0286, found 344.0284.
E-7-[(4-三氟甲基苯基)甲亚基]氨基-3, 4-二氢-苯并[b]氧杂䓬-5(2H)-酮(8b):收率74%, 浅黄色固体. m.p. 88~90 ℃; 1H NMR (400 MHz, CDCl3) δ: 2.27 (p, J=6.7 Hz, 2H), 2.96 (t, J=6.9 Hz, 2H), 4.29 (t, J=6.6 Hz, 2H), 7.15 (d, J=8.6 Hz, 1H), 7.43 (dd, J=8.6, 2.7 Hz, 1H), 7.69 (d, J=2.7 Hz, 1H), 7.75 (d, J=8.2 Hz, 2H), 8.03 (d, J=8.0 Hz, 2H), 8.57 (s, 1H); 13C NMR (125 MHz, CDCl3) δ: 26.3, 40.6, 73.0, 120.1, 121.9, 125.7 (q, 2JCF=3.8 Hz), 125.8, 127.4 (q, 1JCF=270 Hz), 128.1, 129.0, 129.5, 139.1, 146.1, 158.3, 160.9, 200.4; HRMS (ESI) calcd for C18H15NO2F3 [M+H]+ 334.1055, found 334.1064.
E-7-[(4-二甲氨基苯基)甲亚基]氨基-3, 4-二氢-苯并[b]氧杂䓬-5(2H)-酮(8c):收率82%, 浅黄色固体. m.p. 102~104 ℃; 1H NMR (400 MHz, CDCl3) δ: 2.21 (p, J=6.7 Hz, 2H), 2.92 (t, J=6.8 Hz, 2H), 3.05 (s, 6H), 4.24 (t, J=6.6 Hz, 2H), 6.73 (d, J=8.9 Hz, 2H), 7.08 (d, J=8.5 Hz, 1H), 7.35 (dd, J=8.5, 2.6 Hz, 1H), 7.59 (d, J=2.7 Hz, 1H), 7.75 (d, J=8.7 Hz, 2H), 8.35 (s, 1H, 1H); 13C NMR (100 MHz, CDCl3) δ: 160.2, 159.5, 152.5, 148.0, 130.5, 129.5, 128.1, 124.2, 121.7, 119.7, 111.5, 72.8, 40.5, 40.1, 26.1; HRMS (ESI) calcd for C19H21N2O2 [M+H]+ 309.1603, found 309.1606.
E-7-[(3-硝基苯基)甲亚基]氨基-3, 4-二氢-苯并[b]氧杂䓬-5(2H)-酮(8d):收率72%, 浅黄色固体. m.p. 156~158 ℃; 1H NMR (400 MHz, CDCl3) δ: 2.26 (p, J=6.7 Hz, 2H), 2.95 (t, J=6.9 Hz, 2H), 4.29 (t, J=6.6 Hz, 2H), 7.15 (d, J=8.6 Hz, 1H), 7.43 (dd, J=8.6, 2.7 Hz, 1H), 7.65~7.69 (m, 2H), 8.21~8.24 (m, 1H), 8.31~8.34 (m, 1H), 8.59 (s, 1H), 8.74~8.79 (m, 1H); 13C NMR (125 MHz, CDCl3) δ: 25.9, 40.2, 72.7, 119.8, 121.5, 122.9, 125.2, 127.7, 129.1, 129.4, 133.7, 137.3, 145.2, 148.3, 156.4, 160.7, 199.9; HRMS (ESI) calcd for C17H15N2O4[M+H]+ 311.1032, found 311.1036.
E-7-[(2-羟基苯基)甲亚基]氨基-3, 4-二氢-苯并[b]氧杂䓬-5(2H)-酮(8e):收率76%, 浅黄色固体. m.p. 79~81 ℃; 1H NMR (400 MHz, CDCl3) δ: 2.21~2.28 (m, 2H), 2.94 (t, J=6.9 Hz, 2H), 4.27 (t, J=6.6 Hz, 2H), 6.95 (dt, J=1.0, 7.5 Hz, 1H), 7.02 (d, J=8.0 Hz, 1H), 7.14 (d, J=8.6 Hz, 1H), 7.33~7.44 (m, 3H), 7.71 (d, J=2.7 Hz, 1H), 8.65 (s, 1H); 13C NMR (125 MHz, CDCl3) δ: 199.8, 162.0, 160.6, 160.5, 143.0, 132.8, 131.9, 129.2, 127.4, 121.6, 120.0, 118.7, 118.7, 116.8, 72.7, 40.2, 25.8; HRMS (ESI) calcd for C17H16NO3 [M+H]+ 282.1130, found 282.1123.
E-7-[(4-氟-2羟基苯基)甲亚基]氨基-3, 4-二氢-苯并[b]氧杂䓬-5(2H)-酮(8f):收率77%, 浅黄色固体. m.p. 118~120 ℃; 1H NMR (400 MHz, CDCl3) δ: 2.27 (p, J=6.7 Hz, 2H), 2.96 (t, J=7.0 Hz, 2H), 4.30 (t, J=6.6 Hz, 2H), 6.93~7.03 (m, 1H), 7.07~7.20 (m, 3H), 7.42 (dd, J=8.6, 2.8 Hz, 1H), 7.73 (d, J=2.8 Hz, 1H), 8.61 (s, 1H); 13C NMR (100 MHz, CDCl3) δ: 26.3, 40.6, 73.2, 117.2 (d, 2JCF=23.0 Hz), 118.3 (d, 3JCF=7.0 Hz), 118.8 (d, 3JCF=10.0 Hz), 120.4 (d, 2JCF=23.0 Hz), 120.6, 122.1, 127.8, 129.6, 143.0, 155.6 (d, 1JCF=230.0 Hz), 157.2, 161.1 (d, 4JCF=3.0 Hz), 161.3, 200.2; HRMS (ESI) calcd for C17H15NO3F [M+H]+ 300.1036, found 300.1042.
E-7-[(5-溴-2-羟基苯基)甲亚基]氨基-3, 4-二氢-苯并[b]氧杂䓬-5(2H)-酮(8g):收率78%, 浅黄色固体. m.p. 165~166 ℃; 1H NMR (400 MHz, CDCl3) δ: 2.24~2.31 (m, 2H), 2.96 (t, J=6.6 Hz, 2H), 4.29 (t, J=6.6 Hz, 2H), 6.94 (d, J=8.7 Hz, 1H), 7.16 (d, J=8.6 Hz, 1H), 7.40~7.47 (m, 2H), 7.52 (s, 1H), 7.72~7.73 (m, 1H), 8.59 (s, 1H), 13.13 (s, 1H, ); 13C NMR (100 MHz, CDCl3) δ: 26.3, 40.6, 73.2, 110.6, 119.3, 120.5, 120.8, 122.1, 127.6, 129.6, 134.3, 135.8, 142.8, 160.1, 160.8, 161.4, 200.1; HRMS (ESI) calcd for C17H15NO3Br [M+H]+ 360.0235, found 360.0242.
E-7-[(2, 3-二羟基苯基)甲亚基]氨基-3, 4-二氢-苯并[b]氧杂䓬-5(2H)-酮(8h):收率81%, 浅黄色固体. m.p. 132~134 ℃; 1H NMR (400 MHz, CDCl3) δ: 2.27 (p, J=6.7 Hz, 2H), 2.97 (t, J=6.9 Hz, 2H), 4.30 (t, J=6.6 Hz, 2H), 6.86 (t, J=7.8 Hz, 1H), 6.98 (d, J=7.7 Hz, 1H), 7.03~7.11 (m, 1H), 7.16 (d, J=8.6 Hz, 1H), 7.42 (dd, J=8.6, 2.6 Hz, 1H), 7.74 (d, J=2.6 Hz, 1H), 8.65 (s, 1H); 13C NMR (125 MHz, CDCl3) δ: 25.9, 40.2, 72.7, 117.4, 118.1, 118.8, 120.3, 121.7, 122.6, 127.0, 129.2, 142.3, 144.6, 148.5, 160.7, 161.6, 199.8; HRMS (ESI) calcd for C17H16-NO4 [M+H]+ 298.1079, found 298.1088.
E-7-[(3, 4-二羟基苯基)甲亚基]氨基-3, 4-二氢-苯并[b]氧杂䓬-5(2H)-酮(8i):收率83%, 浅黄色固体. m.p. 185~187 ℃; 1H NMR (400 MHz, Acetone-d6) δ: 2.20 (p, J=6.7 Hz, 2H), 2.85 (t, J=6.6 Hz, 2H), 4.26 (t, J=6.6 Hz, 2H), 6.95 (d, J=8.1 Hz, 1H), 7.12 (d, J=8.6 Hz, 1H), 7.32 (dd, J=8.2, 1.9 Hz, 1H), 7.40 (dd, J=8.6, 2.8 Hz, 1H), 7.54 (dd, J=16.1, 2.3 Hz, 2H), 8.46 (s, 1H); 13C NMR (125 MHz, acetone-d6) δ: 25.6, 40.3, 72.6, 114.2, 115.2, 120.5, 121.8, 122.9, 126.7, 129.2, 129.9, 145.4, 147.3, 148.8, 159.5, 159.6, 199.4; HRMS (ESI) calcd for C17H15NO4Na[M+Na]+ 320.0899, found 320.0905.
E-7-[(2, 4-二羟基苯基)甲亚基]氨基-3, 4-二氢-苯并[b]氧杂䓬-5(2H)-酮(8j):收率80%, 浅黄色固体. m.p. 167~169 ℃; 1H NMR (400 MHz, CDCl3) δ: 2.13~2.28 (m, 2H), 2.93 (t, J=6.9 Hz, 2H), 4.27 (t, J=6.6 Hz, 2H), 6.36~6.52 (m, 2H), 7.12 (d, J=8.6 Hz, 1H), 7.27 (d, J=8.1 Hz, 1H), 7.39 (dd, J=8.6, 2.7 Hz, 1H), 7.66 (d, J=2.7 Hz, 1H), 8.58 (s, 1H); 13C NMR (125 MHz, CDCl3) δ: 30.8, 45.2, 77.6, 107.8, 112.7, 117.3, 125.1, 126.7, 132.0, 134.4, 138.8, 148.4, 165.0, 166.4, 166.7, 168.3, 205.0; HRMS (ESI) calcd for C17H16NO4 [M+H]+ 298.1079, found 298.1073.
E-7-[(2, 5-二羟基苯基)甲亚基]氨基-3, 4-二氢-苯并[b]氧杂䓬-5(2H)-酮(8k):收率78%, 浅黄色固体. m.p. 156~158 ℃; 1H NMR (400 MHz, CDCl3) δ: 2.12~2.33 (m, 2H), 2.93 (t, J=6.9 Hz, 2H), 4.28 (t, J=6.6 Hz, 2H), 6.86 (d, J=8.6 Hz, 1H), 6.96 (dd, J=13.5, 2.6 Hz, 2H), 7.14 (d, J=8.6 Hz, 1H), 7.42 (dd, J=8.6, 2.7 Hz, 1H), 7.69 (d, J=2.7 Hz, 1H), 7.78 (s, 1H), 8.60 (s, 1H); 13C NMR (125 MHz, CDCl3) δ: 30.8, 45.3, 77.7, 122.1, 122.4, 123.6, 125.3, 126.0, 126.8, 132.2, 134.3, 148.2, 154.0, 159.2, 165.5, 167.0, 205.0; HRMS (ESI) calcd for C17H16NO4[M+H]+ 298.1079, found 298.1084.
E-7-[(2-羟基-4-甲氧基苯基)甲亚基]氨基-3, 4-二氢-苯并[b]氧杂䓬-5(2H)-酮(8l):收率82%, 浅黄色固体. m.p. 123~125 ℃; 1H NMR (400 MHz, CDCl3) δ: 2.01~2.40 (m, 2H), 2.95 (t, J=6.9 Hz, 2H), 3.86 (s, 3H), 4.28 (t, J=6.6 Hz, 2H), 6.46~6.64 (m, 2H), 7.13 (d, J=8.6 Hz, 1H), 7.24~7.33 (m, 1H), 7.38 (dd, J=8.6, 2.7 Hz, 1H), 7.69 (d, J=2.7 Hz, 1H), 8.57 (s, 1H), 13.58 (s, 1H); 13C NMR (100 MHz, CDCl3) δ: 26.3, 40.6, 55.5, 73.1, 101.1, 107.2, 113.0, 120.2, 122.0, 127.7, 129.6, 133.6, 143.5, 160.6, 161.4, 163.7, 164.0, 200.4; HRMS (ESI) calcd for C18H18NO4[M+H]+ 312.1236, found 312.1228.
E-7-[(5-溴-2-羟基-3-甲氧基苯基)甲亚基]氨基-3, 4-二氢-苯并[b]氧杂䓬-5-(2H)-酮(8m):收率85%, 浅黄色固体. m.p. 145~146 ℃; 1H NMR (400 MHz, CDCl3) δ: 2.27 (p, J=6.7 Hz, 2H), 2.95 (t, J=7.0 Hz, 2H), 3.94 (s, 3H), 4.29 (t, J=6.6 Hz, 2H), 7.07 (d, J=2.2 Hz, 1H), 7.16 (dd, J=5.4, 3.2 Hz, 2H), 7.40 (dd, J=8.6, 2.8 Hz, 1H), 7.72 (d, J=2.7 Hz, 1H), 8.58 (s, 1H); 13C NMR (100 MHz, CDCl3) δ: 26.3, 40.6, 56.4, 73.2, 110.0, 117.7, 119.8, 120.5, 122.2, 125.6, 127.9, 129.6, 142.6, 149.4, 150.6, 161.0, 161.4, 200.1; HRMS (ESI) calcd for C18H17NO4Br [M+H]+ 390.0341, found 390.0333.
E-7-[(2, 3, 4-三羟基苯基)亚基]氨基-3, 4-二氢-苯并[b]氧杂䓬-5-(2H)-酮(8n):收率76%, 浅黄色固体. m.p. 174~176 ℃; 1H NMR (400 MHz, CDCl3) δ: 2.05~2.35 (m, 2H), 2.92 (dd, J=15.6, 8.8 Hz, 2H), 4.26 (dd, J=15.4, 8.9 Hz, 2H), 6.51 (dd, J=17.8, 8.5 Hz, 1H), 6.86 (dd, J=17.9, 8.6 Hz, 1H), 7.11 (dd, J=17.8, 8.6 Hz, 1H), 7.29~7.47 (m, 1H), 7.65 (d, J=15.2 Hz, 1H), 8.53 (s, 1H); 13C NMR (125 MHz, CDCl3) δ: 30.8, 45.2, 77.7, 112.3, 117.3, 125.2, 126.8, 129.0, 131.5, 134.4, 136.7, 147.4, 153.7, 156.4, 165.1, 166.2, 204.9; HRMS (ESI) calcd for C17H16NO5 [M+H]+ 314.1028, found 314.1025.
E-7-[(3, 4, 5-三甲氧基苯基)甲亚基]氨基-3, 4-二氢-苯并[b]氧杂䓬-5(2H)-酮(8o):收率76%, 浅黄色固体. m.p. 120~122 ℃; 1H NMR (400 MHz, CDCl3) δ: 2.25 (p, J=6.7 Hz, 2H), 2.95 (t, J=6.9 Hz, 2H), 3.93 (s, 6H), 3.96 (s, 6H), 4.28 (t, J=6.6 Hz, 2H), 7.10~7.20 (m, 3H), 7.39 (dd, J=8.5, 2.7 Hz, 1H), 7.64 (d, J=2.7 Hz, 1H), 8.42 (s, 1H); 13C NMR (125 MHz, CDCl3) δ: 26.2, 40.6, 56.3, 61.0, 73.0, 105.8, 106.7, 120.0, 121.8, 128.0, 129.5, 131.5, 141.1, 153.5, 159.7, 160.3, 200.5; HRMS (ESI) calcd for C20H22NO5 [M+H]+ 356.1498, found 356.1494.
2.2.3 化合物8k的单晶X衍射分析
室温条件下样品8k在乙醇中缓慢挥发溶剂, 获得黄色透明柱状晶体.选取大小为0.15 mm×0.10 mm×0.10 mm单晶样品, 在BrukeSMART CCD衍射仪上进行晶体结构分析, 用经过石墨单色器单色化的Mo Kα (λ=0.071073 nm)作为入射辐射.最大2θmax=53.98°, 以φ-ω扫描方式, 在296(2) K下共收集12182个衍射点, 其中独立衍射点为3289个, 3289个[I>2σ]可观测衍射点.晶体结构由SHELXT-97程序解出, 主要的晶体学参数为:分子式为C17H15NO4, 分子量为297.30, a=0.57505 (10) nm, b=1.03013 (2) nm, c=1.24336 (3) nm, α=79.531°, β=80.201°, γ=85.957°, V=0.71311(3) nm3, Dm=1.385 g/cm3, Z=2, F(000)=312, 三斜晶系, 空间群为P-1.
2.2.4 化合物8n的单晶X衍射分析
室温条件下样品在乙醇中缓慢蒸发溶剂, 获得黄色透明柱状晶体.选取大小为0.15 mm×0.10 mm×0.10 mm单晶样品, 在Bruke SMART CCD衍射仪上进行晶体结构分析, 用经过石墨单色器单色化的Mo Ka (λ=0.071073 nm)作为入射辐射.最大2θmax=53.98°, 以φ-ω扫描方式, 在296(2) K下共收集21522个衍射点, 其中独立衍射点为3005个, 3005个[I>2σ]可观测衍射点.晶体结构由SHELXT-97程序解出, 主要的晶体学参数为:分子式为C17H14NO5, 计算分子量为297.30, a=1.4000 (3) nm, b=1.1955 (3) nm, c=0.9035 (2) nm, α=90°, β=108.081°, γ=90°, V=1.4376 (6) nm3, Dm=1.443 g/cm3, Z=4, F(000)=652, 单斜晶系, 空间群为P2(1)/c.
2.2.2 化合物9和10的合成
化合物8 (0.5 mmol)溶解于甲醇(3 mL)中, 缓慢加入NaBH4 (19 mg, 0.5mmol), 于室温搅拌30 min, 加入适量水, 乙酸乙酯提取, 硫酸镁干燥, 浓缩, 残余物经硅胶柱层析得化合物9或10.
7-(苯基甲基)氨基-2, 3, 4, 5-四氢-苯并[b]氧杂䓬-5-醇(9a):收率86%, 浅黄色固体. m.p. 100~102 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.85~2.17 (m, 4H), 3.87~3.97 (m, 2H), 4.28 (s, 2H), 4.78 (s, 1H), 6.42 (d, J=8.3 Hz, 1H), 6.66 (s, 1H), 6.84 (t, J=9.4 Hz, 1H), 7.28~7.38 (m, 5H); 13C NMR (125 MHz, CDCl3) δ: 27.0, 33.8, 48.5, 72.4, 73.1, 111.2, 111.4, 121.8, 126.8, 127.2, 128.2, 137.8, 139.0, 144.2, 149.5; HRMS (ESI) calcd for C17H20NO2[M+H]+ 270.1494, found 270.1492.
7-[(4-氟苯基)甲基]氨基-2, 3, 4, 5-四氢-苯并[b]氧杂䓬-5-醇(9b):收率84%, 浅黄色固体. m.p. 122~123 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.92~2.05 (m, 3H), 2.08~2.18 (m, 1H), 3.81~4.08 (m, 2H), 4.28 (s, 2H), 4.70~4.94 (m, 1H), 6.43 (dd, J=8.5, 2.9 Hz, 1H), 6.68 (d, J=2.9 Hz, 1H), 6.86 (d, J=8.5 Hz, 1H), 7.09~7.09 (m, 2H), 7.31~7.41 (m, 2H); 13C NMR (125 MHz, CDCl3) δ: 27.4, 34.3, 48.2, 72.9, 73.6, 111.8 (d, 2JCF=28.75 Hz), 115.5 (d, 3JCF=21.3 Hz), 122.3, 129.1, 129.1, 135.3 (d, 4JCF=3.7 Hz), 138.3, 144.5, 150.0, 162.1 (d, 1JCF=237.5 Hz); HRMS (ESI) calcd for C17H19NO2F [M+H]+ 288.1400, found 288.1392.
7-[(4-氯苯基)甲基]氨基-2, 3, 4, 5-四氢-苯并[b]氧杂䓬-5-醇(9c):收率88%, 浅黄色固体. m.p. 117~119 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.92~1.9 (m, 3H, m, 3H), 2.10~2.16 (m, 1H), 4.29 (s, 2H), 3.80~4.08 (m, 2H), 4.81 (d, J=6.2 Hz, 1H), 6.41 (dd, J=8.4, 2.9 Hz, 1H), 6.67 (d, J=2.8 Hz, 1H), 6.85 (d, J=8.4 Hz, 1H), 7.33 (d, J=7.5 Hz, 4H); 13C NMR (125 MHz, CDCl3) δ: 27.4, 34.3, 48.2, 72.8, 73.5, 111.6, 111.8, 122.3, 128.8, 132.9, 138.0, 138.3, 144.4, 150.1; HRMS (ESI) calcd for C17H19NO2Cl[M+H]+ 304.1104, found 304.1099.
7-[(4-溴苯基)甲基]氨基-2, 3, 4, 5-四氢-苯并[b]氧杂䓬-5-醇(9d):收率90%, 浅黄色固体. m.p. 93~95 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.92~1.97 (m, 3H), 1.98~2.14 (m, 1H), 3.85~3.90 (m, 1H), 3.97~4.03 (m, 1H), 4.27 (s, 2H), 4.81 (dd, J=6.2, 3.5 Hz, 1H), 6.41 (dd, J=8.5, 2.9 Hz, 1H), 6.68 (d, J=2.8 Hz, 1H), 6.85 (d, J=8.4 Hz, 1H), 7.26 (d, J=8.4 Hz, 2H), 7.48~7.42 (d, J=8.4 Hz, 2H); 13C NMR (125 MHz, CDCl3) δ: 27.4, 34.3, 48.3, 72.8, 73.5, 111.7, 111.9, 121.0, 122.3, 129.2, 131.7, 138.3, 138.5, 144.3, 150.1; HRMS (ESI) calcd for C17H19NO2Br[M+H]+ 348.0599, found 348.0590.
7-[(3-硝基苯基)甲基]氨基-2, 3, 4, 5-四氢-苯并[b]氧杂䓬-5-醇(9e):收率78%, 浅黄色油状物. 1H NMR (400 MHz, CDCl3) δ: 1.87~1.96 (m, 3H), 2.04~2.10 (m, 1H), 3.77~4.04 (m, 2H), 4.41 (s, 2H), 4.79 (d, J=7.6 Hz, 1H), 6.36 (dd, J=8.5, 2.9 Hz, 1H), 6.67 (d, J=2.9 Hz, 1H), 6.82 (d, J=8.5 Hz, 1H), 7.50 (t, J=7.9 Hz, 1H), 7.70 (d, J=7.6 Hz, 1H), 8.11 (dd, J=8.2, 1.3 Hz, 1H), 8.23 (s, 1H); 13C NMR (125 MHz, CDCl3) δ: 27.0, 33.9, 47.6, 72.2, 73.1, 111.3, 111.4, 121.7, 121.8, 121.9, 129.1, 132.9, 138.0, 141.6, 143.4, 148.1, 149.8; HRMS (ESI) calcd for C17H19N2O4[M+H]+ 315.1345, found 315.1341.
7-[(4-二甲氨基苯基)甲基]氨基-2, 3, 4, 5-四氢-苯并[b]氧杂䓬-5-醇(9f):收率83%, 浅黄色油状物. 1H NMR (400 MHz, CDCl3) δ: 1.88~2.15 (m, 4H), 2.94 (s, 6H), 3.87~3.97 (m, 2H), 4.17 (s, 2H), 4.80 (dd, J=6.2, 4.6 Hz, 1H), 6.45 (dd, J=8.5, 2.9 Hz, 1H), 6.66 (d, J=2.9 Hz, 1H), 6.70~6.74 (m, 2H), 6.84 (d, J=8.5 Hz, 1H), 7.21~7.27 (m, 2H); 13C NMR (100 MHz, CDCl3) δ: 27.3, 34.1, 40.7, 48.5, 73.0, 73.6, 111.4, 111.8, 112.8, 122.2, 127.1, 128.8, 138.0, 144.9, 149.8, 150.0; HRMS (ESI) calcd for C19H24N2O2Na [M+Na]+ 335.1735, found 335.1729.
7-[(4-三氟甲基苯基)甲基]氨基-2, 3, 4, 5-四氢-苯并[b]氧杂䓬-5-醇(9g):收率85%, 浅黄色固体. m.p. 100~102 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.83~2.20 (m, 4H), 3.77~4.07 (m, 2H), 4.39 (s, 2H), 4.82 (dd, J=6.2, 3.6 Hz, 1H), 6.40 (dd, J=8.5, 2.9 Hz, 1H), 6.69 (d, J=2.9 Hz, 1H), 6.86 (d, J=8.5 Hz, 1H), 7.50 (d, J=8.2 Hz, 2H), 7.61 (d, J=8.1 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 27.4, 34.3, 48.4, 72.8, 73.5, 111.7, 111.8, 122.3, 125.6 (q, 2JCF=3.8 Hz), 126.0 (q, 1JCF=270 Hz), 127.5, 138.3, 143.8, 144.2, 150.2; HRMS (ESI) calcd for C18H19NO2F3[M+H]+ 338.1368, found 338.1360.
7-[(2-羟基苯基)甲基]氨基-2, 3, 4, 5-四氢-苯并[b]氧杂䓬-5-醇(9h):收率87%, 浅黄色固体. m.p. 142~144 ℃; 1H NMR (400 MHz, CDCl3) δ: 2.12~1.71 (m, 4H), 3.68~3.90 (m, 1H), 4.04 (dd, J=10.2, 4.9 Hz, 1H), 4.34 (s, 2H), 4.80 (d, J=8.4 Hz, 1H), 6.61 (dd, J=8.4, 2.8 Hz, 1H), 6.86 (dd, J=8.4, 5.8 Hz, 4H), 7.06~7.40 (m, 2H); 13C NMR (125 MHz, CDCl3) δ: 27.1, 33.9, 48.7, 72.0, 73.0, 114.3, 114.7, 116.1, 119.6, 121.9, 122.6, 128.3, 128.7, 137.9, 142.9, 151.6, 156.3; HRMS (ESI) calcd for C17H20NO3 [M+H]+ 286.1443, found 286.1435.
7-[(4-甲氧基苯基)甲基]氨基-2, 3, 4, 5-四氢-苯并[b]氧杂䓬-5-醇(9i):收率87%, 浅黄色固体. m.p. 84~86 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.85~2.20 (m, 4H), 3.82 (s, 3H), 3.86~3.91 (m, 1H), 3.98~4.03 (m, 1H), 4.23 (s, 2H), 4.81 (dd, J=6.5, 3.3 Hz, 1H), 6.45 (dd, J=8.4, 2.9 Hz, 1H), 6.68 (d, J=2.9 Hz, 1H), 6.86 (d, J=8.4 Hz, 1H), 6.90 (d, J=8.8 Hz, 2H), 7.30 (m, J=8.8 Hz, 2H); 13C NMR (100 MHz, CDCl3) δ: 27.4, 34.2, 48.4, 55.3, 72.9, 73.6, 111.6, 111.8, 114.0, 122.2, 128.9, 131.4, 138.2, 144.7, 149.9, 158.9; HRMS (ESI) calcd for C18H21NO3Na [M+Na]+ 322.1419, found 322.1418.
7-[(4-氟-2-羟基苯基)甲基]氨基-2, 3, 4, 5-四氢-苯并[b]氧杂䓬-5-醇(9j):收率79%, 浅黄色固体. m.p. 114~116 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.84~2.13 (m, 4H), 4.09~3.65 (m, 2H), 4.33 (s, 2H), 4.81 (d, J=8.6 Hz, 1H), 6.61 (dd, J=8.5, 2.8 Hz, 1H), 6.79 (dd, J=8.7, 4.8 Hz, 1H), 6.82~6.95 (m, 4H); 13C NMR (100 MHz, CDCl3) δ: 27.5, 34.3, 49.0, 72.5, 73.4, 114.8 (d, 2JCF=2.0 Hz), 115.1, 115.2, 115.4, 117.4 (d, 2JCF=8.0 Hz), 122.4, 124.0 (d, 3JCF=7.0 Hz), 138.4, 143.0, 152.3, 152.7 (d, 3JCF=2.0 Hz), 156.4 (d, 1JCF=236 Hz); HRMS (ESI) calcd for C17H19NO3F[M+H]+ 304.1349, found 304.1343.
7-[(2-溴-3-羟基苯基)甲基]氨基-2, 3, 4, 5-四氢-苯并[b]氧杂䓬-5-醇(9k):收率80%, 浅黄色固体. m.p. 149~150 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.84~2.14 (m, 4H), 3.82 ~4.02 (m, 2H), 4.34 (s, 2H), 4.76~4. 82 (m, 1H), 6.38 (dd, J=8.5, 2.9 Hz, 1H), 6.65 (d, J=2.9 Hz, 1H), 6.83 (d, J=8.5 Hz, 1H), 6.91~6.99 (m, 2H), 7.15 (t, J=7.8 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ: 26.9, 33.7, 48.8, 72.5, 73.1, 110.9, 111.3, 111.5, 114.5, 120.6, 121.9, 128.1, 137.7, 138.5, 143.7, 149.7, 152.1; HRMS (ESI) calcd for C17H18NO3NaBr [M+Na]+ 386.0368, found 386.0369.
7-[(5-溴-2-羟基苯基)甲基]氨基-2, 3, 4, 5-四氢-苯并[b]氧杂䓬-5-醇(9l):收率81%, 浅黄色固体. m.p. 117~119 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.97 (m, 4H), 3.79~3.84 (m, 1H), 4.02~4.06 (m, 1H, 4.32 (s, 2H), 4.81 (d, J=8.2 Hz, 1H), 6.61 (d, J=8.5 Hz, 1H), 6.74 (d, J=8.6 Hz, 1H), 6.87 (s, 2H), 7.24~7.29 (m, 2H); 13C NMR (125 MHz, CDCl3) δ: 27.0, 33.9, 48.5, 72.0, 73.0, 111.2, 114.5, 114.9, 118.0, 122.0, 124.5, 130.7, 131.4, 138.0, 142.4, 152.0, 155.6; HRMS (ESI) calcd for C17H18NO3Na-Br [M+Na]+ 386.0368, found 386.0376.
7-[(2, 4-二羟基苯基)甲基]氨基-2, 3, 4, 5-四氢-苯并[b]氧杂䓬-5-醇(9m):收率87%, 浅黄色油状物. 1H NMR (400 MHz, DMSO-d6) δ: 1.82~1.88 (m, 4H), 4.00 (s, 2H), 4.57~4.60 (m, 1H), 5.12~5.14 (m, 2H), 6.12 (dd, J=8.2, 2.3 Hz, 1H), 6.22~6.35 (m, 2H), 6.60 (d, J=8.4 Hz, 1H), 6.74 (d, J=2.6 Hz, 1H), 6.94 (d, J=8.4 Hz, 1H), 9.02 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 28.8, 36.1, 42.3, 70.4, 72.8, 102.7, 106.3, 110.4, 111.0, 116.9, 121.3, 129.5, 139.5, 145.4, 148.4, 156.2, 157.3; HRMS (ESI) calcd for C17H17NO4Na [M+Na]+ 322.1055, found 322.1050.
7-[(2-羟基-4-甲氧基苯基)甲基]氨基-2, 3, 4, 5-四氢-苯并[b]氧杂䓬-5-醇(9n):收率83%, 浅黄色油状物, 1H NMR (400 MHz, CDCl3) δ: 1.79~2.14 (m, 4H), 3.79 (s, 3H), 3.82~4.09 (m, 2H), 4.32 (s, 2H), 4.85 (d, J=8.4 Hz, 1H), 6.41~6.50 (m, 2H), 6.66 (dd, J=8.4, 2.9 Hz, 1H), 6.83~6.94 (m, 2H), 7.04 (d, J=8.3 Hz, 1H), 7.23~7.41 (m, 1H); 13C NMR (100 MHz, CDCl3) δ: 27.5, 34.3, 48.8, 55.3, 72.5, 73.5, 102.2, 105.9, 114.9, 115.2, 115.3, 122.3, 129.3, 138.3, 143.4, 152.1, 157.9, 160.6; HRMS (ESI) calcd for C18H22NO4 [M+H]+ 316.1549, found 316.1541.
7-[(5-溴-2-羟基-3-甲氧基苯基)甲基]氨基-2, 3, 4, 5-四氢-苯并[b]氧杂䓬-5-醇(9o):收率82%, 浅黄色固体. m.p. 51~53 ℃; 1H NMR (400 MHz, CDCl3) δ: 2.14~1.83 (m, 4H), 3.80~4.03 (m, 5H), 4.30 (s, 2H), 4.80 (dd, J=6.9, 2.9 Hz, 1H), 6.49 (dd, J=8.5, 2.9 Hz, 1H), 6.73 (d, J=2.8 Hz, 1H), 6.84 (d, J=8.5 Hz, 1H), 6.90 (d, J=2.2 Hz, 1H), 7.01 (d, J=2.2 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ: 26.9, 33.7, 44.5, 55.8, 72.4, 73.1, 110.9, 112.5, 112.6, 1113.0, 21.8, 123.1, 125.7, 137.7, 143.1, 143.5, 147.1, 150.3; HRMS (ESI) calcd for C18H21NO4Br [M+H]+394.0654, found 394.0658.
7-[(3, 4, 5-三甲氧基苯基)甲基]氨基-2, 3, 4, 5-四氢-苯并[b]氧杂䓬-5-醇(9p):收率87%, 浅黄色固体. m.p. 105~107 ℃; 1H NMR (400 MHz, CDCl3) δ: 1.94~1.97 (m, 3H), 2.08~2.16 (m, 1H), 4.23 (s, 2H), 3.86~3.89 (m, 10H), 4.39~4.03 (m, 1H), 4.74~4.89 (m, 1H), 6.45 (dd, J=8.5, 2.9 Hz, 1H), 6.62 (s, 2H), 6.71 (d, J=2.8 Hz, 1H), 6.86 (d, J=8.5 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ: 27.4, 34.3, 49.4, 56.1, 60.9, 72.8, 73.6, 104.5, 111.6, 111.9, 122.3, 135.2, 137.1, 138.2, 144.7, 150.0, 153.4; HRMS (ESI) calcd for C20H25NO5Na[M+H]+382.1630, found 382.1623.
7-[(2, 3-二羟基苯基)甲基]氨基-2, 3, 4, 5-四氢-苯并[b]氧杂䓬-5-醇(10a):收率64%, 浅黄色油状物, 1H NMR (400 MHz, CDCl3) δ: 1.99~2.15 (m, 2H), 2.88 (t, J=6.7 Hz, 2H), 4.15 (t, J=6.7 Hz, 2H), 4.36 (s, 2H), 6.72 (m, 2H), 6.85 (dd, J=7.9, 1.6 Hz, 1H), 6.89~6.99 (m, 2H), 7.22 (d, J=2.6 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ: 25.2, 40.0, 48.0, 72.1, 114.2, 114.9, 119.4, 119.9, 121.7, 121.9, 122.6, 129.3, 142.3, 143.1, 144.4, 155.3, 200.9; HRMS (ESI) calcd for C17H17NO4Na[M+Na]+322.1055, found 322.1058.
7-[(3, 4-二羟基苯基)甲基]氨基-2, 3, 4, 5-四氢-苯并[b]氧杂䓬-5-醇(10b):收率68%, 浅黄色油状物, 1H NMR (400 MHz, CDCl3) δ: 1.96~2.22 (m, 4H), 2.74~2.93 (m, 2H), 3.97~4.20 (m, 4H), 6.69 (dd, J=8.1, 1.8 Hz, 1H), 6.72 (dd, J=8.7, 3.0 Hz, 1H, 1H), 6.76~6.83 (m, 2H), 6.89 (d, J=8.5 Hz, 1H), 6.98 (d, J=3.0 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ: 25.0, 39.9, 47.9, 71.8, 111.2, 114.3, 115.0, 119.5, 119.6, 121.8, 129.4, 131.1, 142.8, 143.6, 143.6, 153.0, 202.3; HRMS (ESI) calcd for C17H17NO4Na [M+Na]+ 322.1055, found 322.1064.
7-[(2, 5-二羟基苯基)甲基]氨基-2, 3, 4, 5-四氢-苯并[b]氧杂䓬-5-醇(10c):收率72%, 浅黄色油状物, 1H NMR (400 MHz, CDCl3) δ: 2.03~2.15 (m, 2H), 2.80~2.89 (m, 2H), 4.12 (t, J=6.7 Hz, 2H), 4.19 (s, 2H), 6.62~6.71 (m, 3H), 6.86 (dd, J=8.6, 2.9 Hz, 1H), 6.92 (d, J=8.6, Hz, 1H), 7.14 (d, J=2.9 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ: 25.5, 40.4, 47.8, 72.4, 114.4, 115.7, 115.8, 117.1, 122.2, 122.3, 124.0, 129.6, 143.0, 149.2, 149.5, 155.3, 202.1; HRMS (ESI) calcd for C17H18NO4 [M+H]+ 300.1236, found 300.1229.
2.2.5 活性测试
酶反应底物Poly(Glu, Tyr) 4:1用无钾离子的PBS (10 mmol/L磷酸钠缓冲液, 150 mmol/L NaCl, pH 7.2~7.4) 稀释成20 μmg/mL, 125 μmL/孔包被酶标板, 置37 ℃反应12~16 h.弃去孔中液体, 洗板, 用T-PBS(含0.1% Tween-20无钾离子的PBS, 200 μmL/孔)洗板三次, 每次5 min.于37℃烘箱中干燥酶标板1~2 h.
每孔加入以反应缓冲液(50 mmol/L HEPES pH 7.4, 50 mmol/L MgCl2, 0.5 mmol/L MnCl2, 0.2 mmol/L Na3VO4, 1 mmol/L DTT)稀释的ATP溶液49 μmL, 每孔中加入1 μL待测试化合物, 再加入50 μL以反应缓冲液稀释的激酶域重组蛋白启动反应, 每次实验需设无ATP对照孔两孔.置37 ℃摇床(100 r/min)反应1 h.弃去孔中液体, T-PBS洗板三次.
加入抗体PY99(抗酪氨酸磷酸化抗体)稀释液(抗体PY99用含BSA 5 mg/mL的T-PBS按体积比1:500稀释), 100 μmL/孔, 37 ℃摇床反应0.5 h, 弃去孔中液体, T-PBS洗板三次.
加入辣根过氧化物酶标记的羊抗鼠二抗稀释液(羊抗鼠二抗用含BSA 5 mg/ml的T-PBS按体积比1:2000稀释), 100 μmL/孔, 37 ℃摇床反应0.5 h.弃去孔中液体, T-PBS洗板三次.
加入2 mg/mL的OPD显色液100 μmL/孔用含有0.03%H2O2的0.1 mol/L柠檬酸-柠檬酸钠缓冲液(pH=5.4) 稀释, 25 ℃避光反应1~10 min.
加入2 mol/L H2SO4 50 μmL/孔中止反应, 用可调波长式微孔板酶标仪VERSAmax读数, 波长为490 nm.
IC50值采用酶标仪随机附带软件以四参数法回归求得, 结果见表 2.
3 结论
本文通过合成得到了一系列新的3, 4-二氢-苯并[b]氧杂䓬-5(2H)-酮和醇了化合物, 结构经NMR和EI-Mass确证, 部分化合物经X单晶衍射确证.通过ELISAs对得到的化合物进行了PTKs抑制活性分析, 结果表明含有邻苯二酚片段的化合物8i和10b对蛋白酪氨酸激酶具有显著的抑制活性, 其中8i对ErbB1和ErbB2的IC50分别为1.0和0.33 μmol/L, 8n对RET的IC50为0.7 μmol/L, 10b对ErbB2的IC50为1.02 μmol/L.
辅助材料(Supporting Information) 化合物8a~8o, 9a~9p和10a~10c的1H NMR、13C NMR谱图, 化合物8k, 8n的单晶数据.这些材料可以免费从本刊网站(http://sioc-journal.cn/)上下载.
-
-
[1]
Lemmon, M. A.; Schlessinge, R. J. Cell 2010, 141, 1117. doi: 10.1016/j.cell.2010.06.011
-
[2]
Kubo, T.; Yamamoto, H. W.; Valencia, I.; Soh, J.; Peyton, M.; Jida, M.; Otani, H.; Fujii, T.; Ouchida, M.; Takigawa, N.; Kiura, K.; Shimizu, K.; Date, H.; Minna, J. D.; Varella-Garcia, M.; Lam, W.L.; Gazdar, A.F.; Toyooka, S. Int. J. Cancer 2015, 124, 1778.
-
[3]
Liu, J.; Sheng, Z.; Zhang, Y.; Li, G. Target. Oncol. 2016, 11, 49. doi: 10.1007/s11523-015-0376-7
-
[4]
Tan, C. S.; Cho, B. C.; Soo, R. A. Lung Cancer 2016, 93, 59. doi: 10.1016/j.lungcan.2016.01.003
-
[5]
Brognard, J.; Hunter, R. T. Curr. Opin. Genet. Dev. 2011, 21, 4. doi: 10.1016/j.gde.2010.10.012
-
[6]
Zamecnikova, A. Expert Opin. Drug Discovery 2014, 9, 77. doi: 10.1517/17460441.2014.865012
-
[7]
Bikker, J. A.; Brooijman, S.; Wissner, A.; Mansour, T. S. J. Med. Chem. 2009, 52, 1493. doi: 10.1021/jm8010542
-
[8]
Newman, D. J.; Cragg, G. M. J. Nat. Prod. 2016, 79, 629. doi: 10.1021/acs.jnatprod.5b01055
-
[9]
Buckle, D. R.; Eggleston, D. S.; Houge-Frydrych, C. S. V.; Pinto, I. L.; Readshaw, S. A.; Smith, D. G.; Webster, R. A. B. J. Chem. Soc., Perkin Trans. 1 1991, 11, 2763.
-
[10]
李凤琼, 穆敏婕, 杨娜, 钟凌, 胡荣, 李晋奇, 白兰, 师健友, 张梅, 有机化学, 2016, 36, 1419. http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract345429.shtmlLi, F.; Mu, M.; Yang, N.; Zhong, L.; Hu, R.; Li, J.; Bai, L.; Shi, J.; Zhang, M. Chin. J. Org. Chem. 2016, 36, 1419 (in Chinese). http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract345429.shtml
-
[11]
Gunatilaka, A. A. L. J. Nat. Prod. 2006, 69, 509. doi: 10.1021/np058128n
-
[12]
Glukhov, A. A.; Kirillov, N. F.; Potapova, A. A.; Makhmudov, R. R., Mardanova, L. G. Pharm. Chem. J. 2010, 44, 483. doi: 10.1007/s11094-010-0497-3
-
[13]
Lloyd, D. G.; Hughes, R. B.; Zisterer, D. M.; Williams, D. C.; Fattorusso, C.; Catalanotti, B.; Campiani, G.; Meegan. M. J. J. Med. Chem. 2004, 47, 5612. doi: 10.1021/jm0495834
-
[14]
Hoeller, U.; Koenig, G. M.; Wright, A. D. Eur. J. Org. Chem. 1999, 2949.
-
[15]
Barrett, I.; Carr, M.; O'Boyle, N.; Greene, L. M.; Knox, A.J.; Lloyd, D. G.; Zisterer, D. M.; Meegan, M. J. J. Enzym. Inhib. Med. Chem. 2010, 25, 180. doi: 10.3109/14756360903169659
-
[16]
Armarego, W. L. F.; Chai, C. L. L. Purification of Laboratory Chemicals, 6th ed., Elsevier Science, Oxford, 2009.
-
[1]
-
表 1 合成的化合物8~10
Table 1. Structure of compounds 8~10

表 2 化合物对PTKs抑制活性a
Table 2. Inhibitory activities against PTKs of the compounds

-

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 4
- 文章访问数: 1164
- HTML全文浏览量: 100


 下载:
下载:


 下载:
下载:

