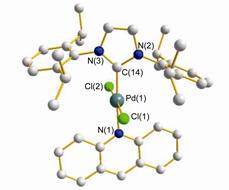
 Figure 1.
Molecular structures of the Pd(Ⅱ) complexes 3a
Figure 1.
Molecular structures of the Pd(Ⅱ) complexes 3a

吖啶作为辅助配体的N-杂环卡宾-钯(Ⅱ)化合物:合成、表征和催化应用
-
关键词:
- N-杂环卡宾
- / 钯
- / 吖啶
- / Suzuki-Miyaura偶联反应
English
N-Heterocyclic Carbene-Palladium(Ⅱ) Complexes with Acridine Ligand:Synthesis, Characterization and Catalytic Applications
-
Key words:
- N-heterocyclic carbene
- / palladium
- / acridine
- / Suzuki-Miyaura cross-coupling
-
The cross-coupling of organic halides and organoboron reagents, known as the Suzuki-Miyaura coupling stands out as one of the most powerful, convenient, and versatile methods to create carbon-carbon bonds and thus has found widespread applications in advanced materials, natural products and organic synthesis.[1~9] Most published examples concern the use of aryl iodides and bromides in the reaction, but the application of aryl chlorides[10~13] has recently attracted much attention mainly due to their economic reason of low cost, availability and stability. Recently, N-heterocyclic carbene (NHC)-palladium complexes, [14~18] as an important and fascinating subclass of palladium catalysts, have been developed and shown good catalytic activity in the Suzuki-Miyaura coupling of aryl chlorides. For example, the PEPPSI (pyridine, enhanced, precatalyst, preparation, stabilization, initiation) NHC-palladium complexes[19~22] readily catalyzed the Suzuki-Miyaura coupling reaction of aryl chlorides with arylboronic acids in good yields. The NHC-Pd(Ⅱ)-Im complexes[23~28] acting as highly effective pre-catalysts can perform the Suzuki-Miyaura coupling of aryl as well as benzyl chlorides with arylboronic acids under mild conditions. Strassner et al.[29] have explored the applications of the NHC-Pd(Ⅱ)-2-phenylimidazole complexes for the Suzuki-Miyaura cross-coupling reaction of aryl chlorides and the reaction was found to tolerate a wide range of substrates at low catalyst loadings. The catalytic activities of imine-Pd-NHC complexes were evaluated for more challenging Suzuki-Miyaura cross-coupling reaction of aryl chlorides.[30] Dinuclear NHC-palladium complexes containing various bridging ligands have also been reported to be active catalyst precursors for the coupling reactions.[31~33] In our previous work, the N-heterocyclic carbene-palladium(Ⅱ) complexes with benzoxazole or benzothiazole ligands were developed, which were used as effective catalysts for the Suzuki-Miyaura coupling of aryl as well as benzyl chlorides with arylboronic acids.[34] Although these species have been found to be particularly useful as catalysts in the Suzuki-Miyaura coupling of aryl chlorides, the development of easily prepared, and highly reactive NHC-Pd(Ⅱ) complexes still constitutes a challenging endeavor in current organometallic chemistry. Encouraged by the results mentioned above and also in continuation of our interest in the construction of functionalized complexes, herein we would like to report the synthesis and structural characterization of N-heterocyclic carbene palladium(Ⅱ) complexes with acridine as ancillary ligands (Eq. 1). The application of the obtained complexes in the Suzuki-Miyaura coupling of aryl and benzyl chlorides with arylboronic acids is also presented below.
1 Results and disscussion
1.1 Synthesis and characterization of the palladium(Ⅱ) complexes
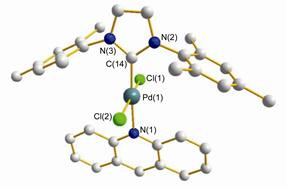
According to our previous report, [34] the synthesis of the required N-heterocyclic carbene-palladium(Ⅱ) complexes 3 was easily done in a one-step sequence from commercially available imidazolium salts, palladium chloride, and acridine as shown in Eq. 1. The expected palladium(Ⅱ) complexes 3 were isolated in good yields after purification and fully characterized by 1H NMR, 13C NMR, and elemental analysis. The molecular structures of Pd complexes 3a and 3b were unambiguously determined by X-ray single crystal analysis. The molecules are illustrated in Figures 1 and 2, respectively. Complexes 3a and 3b showed slightly distorted-square-planar configuration for the central palladium atom. The two chloride anions perpendicular to the plane of the NHC ligands and the acridine is trans to it. All of the bond lengths and angles around the Pd(Ⅱ) center in the two complexes are similar. The values of bond lengths and angles also compare well to those of the related NHC-Pd(Ⅱ) complexes with N-containing compounds.[34] The Pd—N bond lengths (around 2.100 Å) in complexes 3a and 3b are slightly longer than that of Pd—Ccarbene (around 1.975 Å), the Pd—Cl(1) and Pd—Cl(2) bond lengths are nearly identical. The angles of Ccarbene—Pd—N and Cl(1)—Pd—Cl(2) are almost close to 180°, while the Ccarbene—Pd—Cl(1) angles, Ccarbene—Pd—Cl(2) angles, N—Pd—Cl(1) angles and N—Pd—Cl(2) angles are almost close to 90°.
1.2 Suzuki-Miyaura coupling reaction
In order to test the catalytic activities of the N-hetero-cyclic carbene-palladium(Ⅱ) complexes 3a and 3b, initial experiments were carried out using 1-chloro-4-methoxy-benzene and phenylboronic acid as the reactants, plus complex 3a as the catalyst, in i-PrOH-H2O at 80 ℃, with the results shown in Table 2. The choice of base proved to have an important influence on the reaction (Table 2, Entries 1~9).[35] Cs2CO3 was found to be the most effective among the tested bases in this case (Table 2, Entries 6 and 9). When 1.0 mol% complex 3awas tested, the yield decreased (Table 2, Entry 10). In addition, i-PrOH mixed with an equal volume of water was found to be the most appropriate solvent, giving the biaryl product in a > 99% yield (Table 2, Entry 6 vs. Entries 11~13). The complex 3b was less efficient than 3a under identical conditions (Entry 6 vs. Entry 14). Fortunately, in this system, the N-heterocyclic carbene-palladium(Ⅱ) complexes 3 were found to exhibit better catalytic activity compared with a related NHC-Pd(Ⅱ) catalyst (complex Ⅰ or Ⅱ) in the Suzuki-Miyaura coupling reaction. For example, in the presence of 2.0 mol% N-heterocyclic carbene-palladium(Ⅱ) complex Ⅰ, very low yield of the corresponding product 6a was obtained (Entry 15). While similar results were also observed when the palladium(Ⅱ) complex Ⅱ was used as the catalyst (Entry 16). We speculate that the possible reasons were ascribed to the different coordination ability acridine and benzoxazole. However, the other N-heterocyclic carbene-palladium(Ⅱ) complex Ⅲ was examined, and similar yield was also achieved (95% yield, Entry 17).
3a·CH2Cl2 3b·CH2Cl2 Empirical formula C41H47Cl4N3Pd C35H35Cl4N3Pd Mr 830.02 745.86 Temperature/K 301(2) 298(2) Wavelength/Å 0.71073 0.71073 Crystal system Monoclinic Monoclinic Cryst size/mm3 0.35×0.32×0.28 0.28×0.26×0.13 a/Å 13.3144(17) 12.6455(4) b/Å 14.7975(18) 16.2574(5) c/Å 21.804(3) 17.324(6) α/(o) 90 90 β/(o) 103.742(4) 96.6480(10) γ/(o) 90 90 V/Å3 4172.8(9) 3537.2(2) Z 4 4 Space group P2(1)/n P2(1)/c Dcalcd/(g·cm-3) 1.321 1.401 μ/mm-1 0.732 0.854 θ range/(o) 2.92~25.00 2.98~26.00 F(000) 1712 1520 No. of data collected 46724 55414 No. of unique data 7313 6923 R(int) 0.0827 0.0613 Final R indices [I > 2σ(I)] R1=0.1047 R1=0.0517 R indices (all data) R1=0.1342 R1=0.0763  Table 2.
Optimization of reaction conditions for Suzuki-Miyaura reaction of 1-chloro-4-methoxybenzene with phenylboronic acid catalyzed by the NHC-Pd(Ⅱ) complexesa
Table 2.
Optimization of reaction conditions for Suzuki-Miyaura reaction of 1-chloro-4-methoxybenzene with phenylboronic acid catalyzed by the NHC-Pd(Ⅱ) complexesa

Entry Cat. Base Solvent (V:V) Yieldb/% 1 3a KOBu-t i-PrOH/H2O (1:1) > 99 2 3a K2CO3 i-PrOH/H2O (1:1) 89 3 3a K3PO4 i-PrOH/H2O (1:1) 800 4 3a Na2CO3 i-PrOH/H2O (1:1) 88 5 3a NaOBu-t i-PrOH/H2O (1:1) 97 6 3a Cs2CO3 i-PrOH/H2O (1:1) > 99 7 3a NaHCO3 i-PrOH/H2O (1:1) 31 8c 3a KOBu-t i-PrOH/H2O (1:1) 54 9c 3a Cs2CO3 i-PrOH/H2O (1:1) 81 10d 3a Cs2CO3 i-PrOH/H2O (1:1) 89 11 3a Cs2CO3 i-PrOH/H2O (1:2) 74 12 3a Cs2CO3 i-PrOH/H2O (1:3) 48 13 3a Cs2CO3 EtOH/H2O (1:1) 85 14 3b Cs2CO3 i-PrOH/H2O (1:1) 81 15 Ⅰ Cs2CO3 i-PrOH/H2O (1:1) 54 16 Ⅱ Cs2CO3 i-PrOH/H2O (1:1) 35 17 Ⅲ Cs2CO3 i-PrOH/H2O (1:1) 95 aAll reactions were carried out using 4a (0.20 mmol), 5a (0.30 mmol), base (2.0 equiv.), Cat. (2.0 mol%) in solvent (2.0 mL) at 80 ℃ for 3 h. bIsolated yields. cReaction time was 2 h. dCat. (1.0 mol%). With the optimized conditions in hand, a series of aryl chlorides were first used as reactants with phenylboronic acid to test the generality of the reaction. As shown in Table 3, most of the coupling reactions proceeded efficiently to give the corresponding biaryl products 6a~6j in good to excellent yields. Both electron-donating and withdrawing substitutets on the aryl chlorides were tolerated and yields of 79%~ > 99% were obtained for 6a~6h. Whilst the ortho-substituents showed some negative effect on the yields of the catalysis products 6c and 6f. To our pleasure, when heteroaromatic aryl chlorides such as 2-chloropyri-dine and 3-chloropyridine were used as the substrates, high yields of the corresponding products were observed (6i and 6j). Subsequently, the scope of complex 3a catalytic system was further investigated with respect to arylboronic acids (Entries 11~17). In most cases, the reaction worked well and approached to corresponding products in good to almost quantitative yields under identical conditions. However, sterically hindered boronic acid such as 2, 6-dime-thylphenylboronic acid resulted in a significant decrease in yield (100% yield). In addition, in the case of 3-pyridinylboronic acid or 4-pyridinylboronic acid afforded trace amounts of product under the present reaction conditions (data not shown in Table 3). Overview, the results indicated that complex 3a was still efficient in the catalytic process.
 Table 3.
Substrate scope for the catalytic Suzuki-Miyaura reaction of aryl chlorides using the NHC-Pd(Ⅱ) complex 3a as the catalysta
Table 3.
Substrate scope for the catalytic Suzuki-Miyaura reaction of aryl chlorides using the NHC-Pd(Ⅱ) complex 3a as the catalysta

Entry Ar1 Ar2 Product Yieldb/% 1 4-MeOC6H4 Ph 6a > 99 2 3-MeOC6H4 Ph 6b 99 3 2-MeOC6H4 Ph 6c 93 4 4-MeC6H4 Ph 6d 99 5 3-MeC6H4 Ph 6e 95 6 2-MeC6H4 Ph 6f 79 7 4-CH3COC6H4 Ph 6g 99 8 4-O2NC6H4 Ph 6h 99 9 2-Pyridyl Ph 6i 98 10 3-Pyridyl Ph 6j 86 11 4-MeOC6H4 4-MeC6H4 6k 99 12 4-MeOC6H4 3-MeC6H4 6l 96 13 4-MeOC6H4 2-MeC6H4 6m 91 14 4-MeOC6H4 4-FC6H4 6n 99 15 4-MeOC6H4 4-CF3C6H4 6o 98 16 4-MeOC6H4 1-Naphthyl 6p 91 17 4-MeOC6H4 2-Naphthyl 6q 99 aAll reactions were carried out using 4 (0.20 mmol), 5 (0.30 mmol), Cs2CO3 (2.0 equiv.), Cat. 3a (2.0 mol%) in i-PrOH/H2O [V:V=1:1 (2.0 mL)] at 80 ℃ for 3 h. bIsolated yields. Inspired by these successful results, we then turned our interest to such transformations using the benzyl chlorides as the substrates. As shown in Table 4, all reactions proceed smoothly to afford diarylmethanes in good to almost quantitative yields under identical conditions. Particularly, when sterically hindered boronic acids such as 2-methylphenylboronic acid were used as the substrates, high yield of the corresponding product was always observed (99% yield, Entry 3). Overview, the above results confirm that the present N-heterocyclic carbene-palladium(Ⅱ) complexes are highly efficient catalysts for the Suzuki-Miyaura coupling of aryl as well as benzyl chlorides with arylboronic acids.
 Table 4.
Substrate scope for the catalytic Suzuki-Miyaura reaction of benzyl chlorides using the NHC-Pd(Ⅱ) complex 3a as the catalysta
Table 4.
Substrate scope for the catalytic Suzuki-Miyaura reaction of benzyl chlorides using the NHC-Pd(Ⅱ) complex 3a as the catalysta

Entry Ar3 Ar2 Product Yieldb/% 1 Ph 4-MeC6H4 8a > 99 2 Ph 3-MeC6H4 8b 99 3 Ph 2-MeC6H4 8c 99 5 Ph 4-FC6H4 8d 99 6 Ph 4-CF3C6H4 8e 98 7 Ph 1-Naphthyl 8f 96 8 4-t-BuC6H4 Ph 8g 99 9 4-MeC6H4 Ph 8h 99 10 3-MeC6H4 Ph 8i 99 11 2-MeC6H4 Ph 8j 99 12 4-FC6H4 Ph 8k 98 aAll reactions were carried out using 7 (0.20 mmol), 5 (0.30 mmol), Cs2CO3 (2.0 equiv), Cat. 3a (2.0 mol%) in i-PrOH/H2O [V:V=1:1 (2.0 mL)] at 80 ℃ for 3 h. bIsolated yields. 2 Conclusion
In summary, the easily available, well-defined N-hetero-cyclic carbene-palladium(Ⅱ) complexes 3a and 3b, being derived from the corresponding imidazolium salts, palladium chloride and acridine, showed good catalytic activity in the Suzuki-Miyaura coupling of aryl chlorides and benzyl chlorides with arylboronic acids. Further ex ploration of these N-heterocyclic carbene-palladium(Ⅱ) complexes and their catalytic applications in other reactions is in progress.
3 Experimental
3.1 Apparatus and reagents
Melting points were measured on a XT4A melting point apparatus and uncorrected. 1H NMR and 13C NMR spectra were recorded on a Bruker DPX 400 instrument using TMS as an internal standard. Elemental analyses were measured on a Thermo Flash EA 1112 elemental analyzer. Reactions for the preparation of N-heterocyclic carbene-palladium(Ⅱ) complexes were carried out under nitrogen atmosphere. Solvents were dried with standard methods and freshly distilled prior to use if needed. All other chemicals were used as purchased.
3.2 Synthesis of N-heterocyclic carbene-palladium (Ⅱ) complexes
Under an N2 atmosphere, the mixture of imidazolium salts (1.1 mmol), acridine (2.0 mmol, 358.4 mg), PdCl2 (1.0 mmol, 177.3 mg) and K2CO3 (1.1 mmol, 152.0 mg) was stirred in anhydrous tetrahydrofuran (THF) (10 mL) under reflux for 16 h. After cooling, filtration and evaporation, the residue was purified by preparative thin layer chromatography (TLC) on silica gel plates eluting with CH2Cl2 to afford the corresponding N-heterocyclic carbene-palladium(Ⅱ) complexes 3a and 3b.
trans-[1, 3-Bis(2, 6-diisopropylphenyl)imidazol-2-ylidene]-(acridine)-palladium(Ⅱ) dichloride (3a): 67% yield, orange solids. m.p. 143~145 ℃; 1H NMR (400 MHz, CDCl3) δ: 9.17 (d, J=8.7 Hz, 2H, ArH), 8.61 (s, 1H, ArH), 7.77 (d, J=5.2 Hz, 2H, ArH), 7.71~7.67 (m, 2H, ArH), 7.55~7.54 (m, 6H, ArH), 7.40 (s, 2H, ArH), 7.29 (s, 2H, ArH), 3.32~3.30 [m, 4H, CH(CH3)2], 1.44 (d, J=5.8 Hz, 12H, CH3CHCH3), 1.15 (d, J=6.1 Hz, 12H, CH3CHCH3); 13C NMR (100 MHz, CDCl3) δ: 158.6, 147.6, 147.5, 138.7, 135.3, 131.2, 130.2, 128.9, 127.9, 127.2, 125.8, 124.8, 123.9, 29.0, 26.7, 22.6; IR (KBr) ν: 3118, 3085, 2960, 2925, 2865, 1621, 1522, 1460, 1442, 1383, 1363, 1348, 1333, 1268, 926, 800, 756, 731, 707, 602, 499 cm-1; MS (ESI+) m/z: 708.1 (M-Cl). Anal. calcd for C40H45Cl2N3Pd: C 64.48, H 6.09, N 5.64; found C 64.43, H 6.10, N 5.66.
trans-[1, 3-Bis(2, 4, 6-trimethylphenyl)imidazol-2-ylidene]-(acridine)-palladium(Ⅱ) dichloride (3b): 55% yield, orange solids. m.p. 135~138 ℃; 1H NMR (400 MHz, CDCl3) δ: 9.15 (d, J=8.7 Hz, 2H, ArH), 8.66 (s, 1H, ArH), 7.82 (d, J=8.0 Hz, 2H, ArH), 7.56 (t, J=7.4 Hz, 2H, ArH), 7.45 (t, J=6.9 Hz, 2H, ArH), 7.25 (s, 6H, ArH), 2.56 (s, 6H, CH3), 2.46 (s, 12H, CH3); 13C NMR (100 MHz, CDCl3)δ: 156.6, 147.7, 139.2, 138.8, 137.2, 135.2, 131.4, 129.2, 128.5, 128.0, 127.2, 125.9, 123.8, 21.4, 19.3; IR (KBr) ν: 3160, 3127, 2917, 1620, 1570, 1521, 1484, 1460, 1411, 1369, 1339, 1279, 1231, 1154, 1038, 1010, 910, 861, 783, 733, 707, 601 cm-1; MS (ESI+) m/z: 624.0 (M-Cl)+. Anal. calcd for C34H33Cl2N3Pd: C 61.78, H 5.03, N 6.36; found C 61.73, H 5.10, N 6.39.
3.3 General procedure for the catalytic Suzuki-Miyaura reaction
A Schlenk flask was charged with aryl chlorides (0.20 mmol), arylboronic acids (0.30 mmol), N-heterocyclic carbene-palladium(Ⅱ) complex 3 (2.0 mol%), Cs2CO3 (2.0 equiv., 651.6 mg), i-PrOH (1.0 mL) and H2O (1.0 mL). The mixture was stirred at 80 ℃ for 3 h. After cooling, the reaction mixture was evaporated and the product was isolated by preparative TLC on silica gel plates.
3.4 Crystal structure determination and data co-llection
Crystals of 3a and 3b were obtained by recrystallization from CH2Cl2/n-hexane at ambient temperature. Their data were collected on an Oxford Diffraction Gemini E diffractometer with graphite-monochromated Mo Kα radiation (λ=0.7107 Å). The structures were solved by direct methods using the SHELXS-97 program, and all non-hydrogen atoms were refined anisotropically on F2 by the full-matrix least-squares technique, which used the SHELXL-97 crystallographic software package.[36, 37] The hydrogen atoms were included but not refined. Details of the crystal structure determination of the Pd(Ⅱ) complexes are summarized in Table S1 in the Supporting Information. CCDCs 1499311 and 1499312 contain the crystallographic data for complexes 3a and 3b, respectively.
Supporting Information 1H NMR, 13C NMR spectra of compounds 3a and 3b and the 1H NMR spectra of catalysis products. The Supporting Information is available free of charge via the Internet at http://sioc-journal.cn.
-
-
[1]
Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457. doi: 10.1021/cr00039a007
-
[2]
Suzuki, A. J. Organomet. Chem. 1999, 576, 147. doi: 10.1016/S0022-328X(98)01055-9
-
[3]
Corbet, J.-P.; Mignani, G. Chem. Rev. 2006, 106, 2651. doi: 10.1021/cr0505268
-
[4]
姜岚, 李争宁, 赵德峰, 有机化学, 2010, 30, 200. http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract338643.shtmlJiang, L.; Li, Z.; Zhao, D. Chin. J. Org. Chem. 2010, 30, 200(in Chinese). http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract338643.shtml
-
[5]
Zhang, G.; Zhang, W.; Luan, Y.; Han, X.; Ding, C. Chin. J. Chem. 2015, 33, 705. doi: 10.1002/cjoc.v33.7
-
[6]
Liu, C.; Liu, G.; Zhao, H. Chin. J. Chem. 2016, 34, 1048. doi: 10.1002/cjoc.v34.10
-
[7]
李清寒, 丁勇, 张刚, 张震, 莫松, 有机化学, 2016, 36, 83. http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract345224.shtmlLi, Q.; Ding, Y.; Zhang, G.; Zhang, Z.; Mo, S. Chin. J. Org. Chem. 2016, 36, 83(in Chinese). http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract345224.shtml
-
[8]
袁定重, 张庆华, 廖世军, 熊文文, 元利刚, 蔡奇胜, 杨梦梅, 李雄, 蒋烨佳, 刘妍, 李萍, 徐贞帅, 孙盼盼, 耿会玲, 有机化学, 2015, 35, 961. http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract344764.shtmlYuan, D.; Zhang, Q.; Liao, S.; Xiong, W.; Yuan, L.; Cai, Q.; Yang, M.; Li, X.; Jiang, Y.; Liu, Y.; Li, P.; Xu, Z.; Sun, P.; Geng, H. Chin. J. Org. Chem. 2015, 35, 961(in Chinese). http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract344764.shtml
-
[9]
Gu, N.; Liu, Y.; Liu, P.; Ma, X.; Liu, Y.; Dai, B. Chin. J. Chem. 2015, 33, 1189. doi: 10.1002/cjoc.201500446
-
[10]
Buchwald, S. L.; Wolfe, J. P.; Old, D. W. J. Am. Chem. Soc. 1998, 120, 9722. doi: 10.1021/ja982250+
-
[11]
Littke, A. F.; Fu, G. C. Angew. Chem., Int. Ed. 1998, 37, 3387. doi: 10.1002/(ISSN)1521-3773
-
[12]
Sau, S. C.; Santra, S.; Sen, T. K.; Mandal, S. K.; Koley, D. Chem. Commun. 2012, 48, 555. doi: 10.1039/C1CC15732A
-
[13]
Yuan, B.; Pan, Y.; Li, Y.; Yin, B.; Jiang, H. Angew. Chem., Int. Ed. 2010, 49, 4054. doi: 10.1002/anie.201000576
-
[14]
Diez-Gonzalez, S.; Marion, N.; Nolan, S. P. Chem. Rev. 2009, 109, 3612. doi: 10.1021/cr900074m
-
[15]
Droge, T.; Glorius, F. Angew. Chem., Int. Ed. 2010, 49, 6940. doi: 10.1002/anie.201001865
-
[16]
Fortman, G. C.; Nolan, S. P. Chem. Soc. Rev. 2011, 40, 5151. doi: 10.1039/c1cs15088j
-
[17]
Budagumpi, S.; Haque, R. A.; Salman, A. W. Coord. Chem. Rev. 2012, 256, 1787. doi: 10.1016/j.ccr.2012.04.003
-
[18]
唐演, 杨飞飞, 聂士鹏, 王林, 罗治斌, 陆鸿飞, 有机化学, 2015, 35, 705. http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract344694.shtmlTang, Y.; Yang, F.; Nie, S.; Wang, L.; Luo, Z.; Lu, H. Chin. J. Org. Chem. 2015, 35, 705(in Chinese). http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract344694.shtml
-
[19]
Tu, T.; Sun, Z.; Fang, W.; Xu, M.; Zhou, Y. Org. Lett. 2012, 14, 4250. doi: 10.1021/ol3019665
-
[20]
Teci, M.; Brenner, E.; Matt, D.; Toupet, L. Eur. J. Inorg. Chem. 2013, 2841.
-
[21]
Rajabi, F.; Thiel, W. R. Adv. Synth. Catal. 2014, 356, 1873. doi: 10.1002/adsc.201300841
-
[22]
Dunsford, J. J.; Cavell, K. J. Organometallics 2014, 33, 2902. doi: 10.1021/om5003107
-
[23]
Lv, H.; Zhu, L.; Tang, Y.-Q.; Lu, J.-M. Appl. Organomet. Chem. 2014, 28, 27. doi: 10.1002/aoc.3053
-
[24]
Zhang, Y.; Feng, M.-T.; Lu, J.-M. Org. Biomol. Chem. 2013, 11, 2266. doi: 10.1039/c3ob27353a
-
[25]
Wang, Z.-Y.; Chen, G.-Q.; Shao, L.-X. J. Org. Chem. 2012, 77, 6608. doi: 10.1021/jo301270t
-
[26]
Yin, H.-Y.; Liu, M.-Y.; Shao, L.-X. Org. Lett. 2013, 15, 6042. doi: 10.1021/ol4029447
-
[27]
Gu, Z.-S.; Chen, W.-X.; Shao, L.-X. J. Org. Chem. 2014, 79, 5806. doi: 10.1021/jo5010058
-
[28]
Shen, X.-B.; Zhang, Y.; Chen, W.-X.; Xiao, Z.-K.; Hu, T.-T.; Shao, L.-X. Org. Lett. 2014, 16, 1984. doi: 10.1021/ol500531m
-
[29]
Micksch, M.; Tenne, M.; Strassner, T. Organometallics 2014, 33, 3966. doi: 10.1021/om5004336
-
[30]
Shen, A.; Ni, C.; Cao, Y.-C.; Zhou, H.; Song, G.-H.; Ye, X.-F. Tetrahedron Lett. 2014, 55, 3278. doi: 10.1016/j.tetlet.2014.04.044
-
[31]
Yang, J.; Wang, L. Dalton Trans. 2012, 41, 12031. doi: 10.1039/c2dt31174g
-
[32]
Yang, J.; Li, P.; Zhang, Y.; Wang, L. J. Organomet. Chem. 2014, 766, 73. doi: 10.1016/j.jorganchem.2014.05.001
-
[33]
Yang, J.; Li, P.; Zhang, Y.; Wang, L. Dalton Trans. 20014, 43, 7166.
-
[34]
Wang, T.; Xie, H.; Liu, L.; Zhao, W.-X. J. Organomet. Chem. 2016, 804, 73. doi: 10.1016/j.jorganchem.2015.12.039
-
[35]
欧阳昆冰, 席振峰, 化学学报, 2013, 71, 13.Ouyang, K.; Xi, Z. Acta Chim. Sinica 2013, 71, 13(in Chinese).
-
[36]
Sheldrick, G. M. SHELXS-97, Program for Crystal Structure Solution, University of Göttingen, Göttingen, Germany, 1997.
-
[37]
Sheldrick, G. M. SHELXL-97, Program for Crystal Structure Refinement, University of Göttingen, Göttingen, Germany, 1997.
-
[1]
-
Figure 1 Molecular structures of the Pd(Ⅱ) complexes 3a
Hydrogen atoms and solvent molecules are omitted for clarity. Selected bond lengths (Å) and angles (°) in complex 3a: Pd(1)—C(14) 1.981(10), Pd(1)—N(1) 2.109(10), Pd(1)—Cl(1) 2.313(3), Pd(1)—Cl(2) 2.290 (3); C(14)—Pd(1)—Cl(1) 91.6(3), N(1)—Pd(1)—Cl(1) 88.7(3), C(14)—Pd(1)—Cl(2) 90.2(3), N(1)—Pd(1)—Cl(2) 89.7(3), C(14)—Pd(1)—N(1) 176.7(4), Cl(1)—Pd(1)—Cl(2) 175.73(13).
Figure 2 Molecular structures of the Pd(Ⅱ) complexes 3b
Hydrogen atoms and solvent molecules are omitted for clarity. Selected bond lengths (Å) and angles (°) in complex 3b: Pd(1)—C(14) 1.972(4), Pd(1)—N(1) 2.095(3), Pd(1)—Cl(1) 2.3005(12), Pd(1)—Cl(2) 2.2993(12); C(14)—Pd(1)—Cl(1) 90.30(11), N(1)—Pd(1)—Cl(1) 87.54(10), C(14)—Pd(1)—Cl(2) 93.16(11), N(1)—Pd(1)—Cl(2) 89.11(10), C(14)—Pd(1)—N(1) 176.66(15), Cl(1)—Pd(1)—Cl(2) 175.81(5).
Table 1. Summary of crystallographic details for complexes 3a annd 3b
3a·CH2Cl2 3b·CH2Cl2 Empirical formula C41H47Cl4N3Pd C35H35Cl4N3Pd Mr 830.02 745.86 Temperature/K 301(2) 298(2) Wavelength/Å 0.71073 0.71073 Crystal system Monoclinic Monoclinic Cryst size/mm3 0.35×0.32×0.28 0.28×0.26×0.13 a/Å 13.3144(17) 12.6455(4) b/Å 14.7975(18) 16.2574(5) c/Å 21.804(3) 17.324(6) α/(o) 90 90 β/(o) 103.742(4) 96.6480(10) γ/(o) 90 90 V/Å3 4172.8(9) 3537.2(2) Z 4 4 Space group P2(1)/n P2(1)/c Dcalcd/(g·cm-3) 1.321 1.401 μ/mm-1 0.732 0.854 θ range/(o) 2.92~25.00 2.98~26.00 F(000) 1712 1520 No. of data collected 46724 55414 No. of unique data 7313 6923 R(int) 0.0827 0.0613 Final R indices [I > 2σ(I)] R1=0.1047 R1=0.0517 R indices (all data) R1=0.1342 R1=0.0763 Table 2. Optimization of reaction conditions for Suzuki-Miyaura reaction of 1-chloro-4-methoxybenzene with phenylboronic acid catalyzed by the NHC-Pd(Ⅱ) complexesa

Entry Cat. Base Solvent (V:V) Yieldb/% 1 3a KOBu-t i-PrOH/H2O (1:1) > 99 2 3a K2CO3 i-PrOH/H2O (1:1) 89 3 3a K3PO4 i-PrOH/H2O (1:1) 800 4 3a Na2CO3 i-PrOH/H2O (1:1) 88 5 3a NaOBu-t i-PrOH/H2O (1:1) 97 6 3a Cs2CO3 i-PrOH/H2O (1:1) > 99 7 3a NaHCO3 i-PrOH/H2O (1:1) 31 8c 3a KOBu-t i-PrOH/H2O (1:1) 54 9c 3a Cs2CO3 i-PrOH/H2O (1:1) 81 10d 3a Cs2CO3 i-PrOH/H2O (1:1) 89 11 3a Cs2CO3 i-PrOH/H2O (1:2) 74 12 3a Cs2CO3 i-PrOH/H2O (1:3) 48 13 3a Cs2CO3 EtOH/H2O (1:1) 85 14 3b Cs2CO3 i-PrOH/H2O (1:1) 81 15 Ⅰ Cs2CO3 i-PrOH/H2O (1:1) 54 16 Ⅱ Cs2CO3 i-PrOH/H2O (1:1) 35 17 Ⅲ Cs2CO3 i-PrOH/H2O (1:1) 95 aAll reactions were carried out using 4a (0.20 mmol), 5a (0.30 mmol), base (2.0 equiv.), Cat. (2.0 mol%) in solvent (2.0 mL) at 80 ℃ for 3 h. bIsolated yields. cReaction time was 2 h. dCat. (1.0 mol%). Table 3. Substrate scope for the catalytic Suzuki-Miyaura reaction of aryl chlorides using the NHC-Pd(Ⅱ) complex 3a as the catalysta

Entry Ar1 Ar2 Product Yieldb/% 1 4-MeOC6H4 Ph 6a > 99 2 3-MeOC6H4 Ph 6b 99 3 2-MeOC6H4 Ph 6c 93 4 4-MeC6H4 Ph 6d 99 5 3-MeC6H4 Ph 6e 95 6 2-MeC6H4 Ph 6f 79 7 4-CH3COC6H4 Ph 6g 99 8 4-O2NC6H4 Ph 6h 99 9 2-Pyridyl Ph 6i 98 10 3-Pyridyl Ph 6j 86 11 4-MeOC6H4 4-MeC6H4 6k 99 12 4-MeOC6H4 3-MeC6H4 6l 96 13 4-MeOC6H4 2-MeC6H4 6m 91 14 4-MeOC6H4 4-FC6H4 6n 99 15 4-MeOC6H4 4-CF3C6H4 6o 98 16 4-MeOC6H4 1-Naphthyl 6p 91 17 4-MeOC6H4 2-Naphthyl 6q 99 aAll reactions were carried out using 4 (0.20 mmol), 5 (0.30 mmol), Cs2CO3 (2.0 equiv.), Cat. 3a (2.0 mol%) in i-PrOH/H2O [V:V=1:1 (2.0 mL)] at 80 ℃ for 3 h. bIsolated yields. Table 4. Substrate scope for the catalytic Suzuki-Miyaura reaction of benzyl chlorides using the NHC-Pd(Ⅱ) complex 3a as the catalysta

Entry Ar3 Ar2 Product Yieldb/% 1 Ph 4-MeC6H4 8a > 99 2 Ph 3-MeC6H4 8b 99 3 Ph 2-MeC6H4 8c 99 5 Ph 4-FC6H4 8d 99 6 Ph 4-CF3C6H4 8e 98 7 Ph 1-Naphthyl 8f 96 8 4-t-BuC6H4 Ph 8g 99 9 4-MeC6H4 Ph 8h 99 10 3-MeC6H4 Ph 8i 99 11 2-MeC6H4 Ph 8j 99 12 4-FC6H4 Ph 8k 98 aAll reactions were carried out using 7 (0.20 mmol), 5 (0.30 mmol), Cs2CO3 (2.0 equiv), Cat. 3a (2.0 mol%) in i-PrOH/H2O [V:V=1:1 (2.0 mL)] at 80 ℃ for 3 h. bIsolated yields. -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 7
- 文章访问数: 1385
- HTML全文浏览量: 148


 下载:
下载:

 下载:
下载:

