 Figure Figure1.
Structures of compounds 1~4
Figure Figure1.
Structures of compounds 1~4

阿拉华蜡孔菌中四个新的2,5-二取代呋喃邻二醇
English
Four New 2, 5-Disubstituted Furan Vicinal Diols from the Fungus Ceriporia alachuana
-
The vicinal diol group widely present in many types of natural products, e.g. saccharides, vitamins, nucleotides, steriods, sphingolipids, macrolides and terpenoids, is an important functional group for maintaining biological activities.[1~4] In organic chemistry, vicinal diols are important chiral synthesis building blocks which can be used as valuable controlling structures in asymmetric synthesis.[5] However, the determination of the absolute configuration of vicinal diols is not an easy work even though state-of-the-art instruments have been widely adopted in natural product chemistry. In 1972, Harada and Nakanish[6] reported the exciton chirality method to determine the absolute configurations of a series of cyclic vicinal diols by circular dichroism spectrum. However, this method was proven to be inefficient on acyclic vicinal diols since several conformers with different spectroscopic properties coexisted in solution.[7] In order to determine acyclic vicinal diols, various alternative techniques were established, mainly exploiting optical properties of transition metal complex.[8~13] The method employing dimolybdenum tetraacetate [Mo2-(OAc)4] is regarded as the most convenient and reliable, which can be used for assigning the absolute configuration of natural vicinal diols.[14, 15]
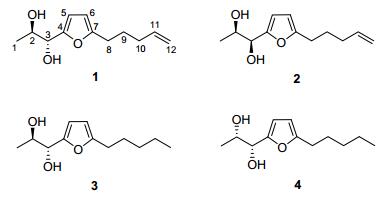
In this paper, we apply the Mo2(OAc)4 induced circular dichroism (ICD) to determine the absolute configuration of four new 2, 5-disubstituted furan vicinal diols, namely, (1R, 2R)-1-(5-(pent-4-en-1-yl)furan-2-yl)propane-1, 2-diol (1), (1S, 2R)-1-(5-(pent-4-en-1-yl)furan-2-yl)propane-1, 2-diol (2), (1R, 2R)-1-(5-pentylfuran-2-yl)propane-1, 2-diol (3) and (1R, 2S)-1-(5-pentylfuran-2-yl)propane-1, 2-diol (4), which were isolated from the cultures of the fungus Ceriporia alachuana (Figure 1).
1 Results and discussion
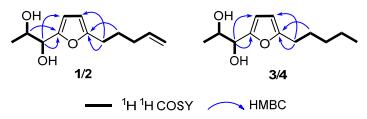
Compound 1 was assigned with a molecular formula of C12H18O3 according to HREIMS spectrum at m/z 210.1257 (calcd for C12H18O3 210.1256). In accordance with its mo-lecular formula, all the 12 carbons including one methyl, four methylenes, three methines and two quaternary carbons were well resolved in the 13C NMR (DEPT) spectrum. One 2, 5-disubstituted furan moiety was constructed from the proton signals at δH 6.16 (d, J=2.8 Hz, 1H) and 5.96 (d, J=2.8 Hz, 1H), as well as the characteristic carbons at δC 155.4 (C-4), 107.8 (C-5), 106.2 (C-6) and 155.1 (C-7). The protons at δH 5.83 (m, 1H), 5.02 (d, J=17.1 Hz, 1H) and 4.95 (d, J=10.2 Hz, 1H) in the 1H NMR spectrum manifested a terminal double bond. In the 1H-1H COSY spectrum, the consecutive correlations of H-8/H-9/H-10/H-11/ H-12 established the pent-4-enyl group, which was further determined to be linked with furan moiety by C(7)—C(8) based on the HMBC correlations from H-8 to C-6, from H-9 to C-7 and from H-6 to C-8. Similarly, apropane-1, 2-diol group was manifested from the 1H-1H COSY correlations of OH-2/H-2, OH-3/H-3 and H-1/H-2/H-3. The linkage between C-3 and C-4 was deduced from the correlation from H-2 to C-4, and from H-3 to C-4 and C-5 in the HMBC spectrum. Therefore, the planar structure of 1 was established to be 1-(5-(pent-4-en-1-yl)furan-2-yl)propane-1, 2-diol as shown in Figure 2.
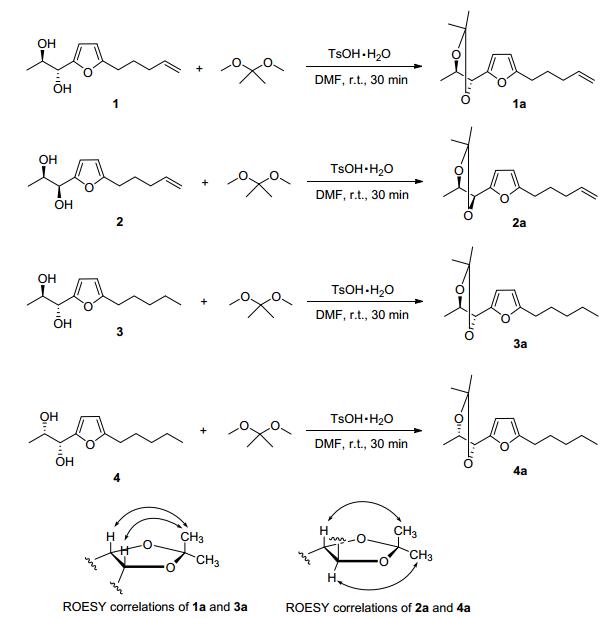
In order to determine the relative stereochemistry, compound 1 was reacted with 2, 2-dimethoxypropane to yield its di-O-isopropylidene derivative 1a. Subsequent ROESY experiment of 1a manifested that H-1 and H-2 showed correlation with the same methyl on isopropyl.[16] Therefore compound 1 was deduced to be an erythro-form vicinal diol (Scheme 1).
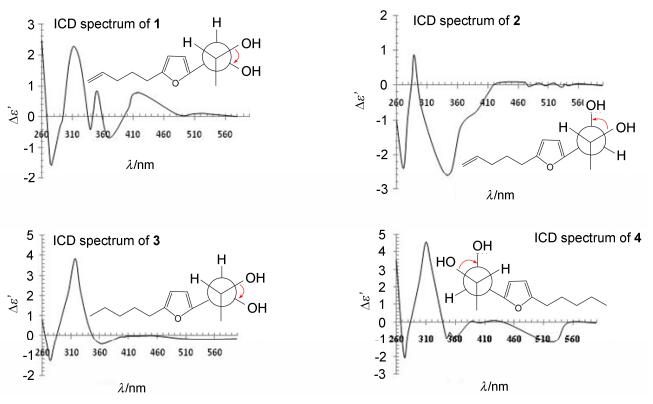
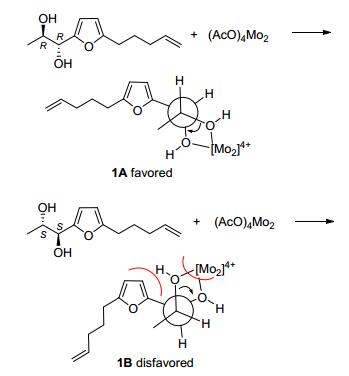
With the characterization of the relative configuration, dimolybdenum tetraacetate [Mo2(OAc)4] induced CD (ICD) spectrum was applied to determine the absolute configuration of 1.[17] According to the Snatzke's rule, the positive torsional angle of the O—C—C—O moiety leads to the positive Cotton effect at about 310 nm, and vice versa. As shown in Figure 3, the ICD spectrum of 1 showed a positive Cotton effect at 300~350 nm, suggesting a positive torsional angle of the O—C—C—O moiety. In order to form a positive torsional angle, the 2R, 3R-form could maintain the favored conformation (1A) in which the bulky furan and O—C—C—O center stayed away from each other; however, for the 2S, 3S-form (1B), the two bulky groups should be adjacent with high steric hindrance, which is unfavorable (Scheme 2). From the above analysis, the absolute configuration of 1 was determined to be 2R, 3R.
Compound 2 shared the same HREIMS and almost identical 1D and 2D NMR spectra with 1, revealing the same planar structure. The minor difference in their NMR spectra, mainly on C-1, C-2 and C-3 positions, should be ascribed to the structural variation of the vicinal diol group. To determine the stereochemistry of the diol, corresponding di-O-isopropylidene derivative (2a) was synthesized and its ROESY experiment was performed. Different from 1a, H-1 and H-2 showed obvious correlations with different methyls of the isopropyl moiety in the REOSY spectrum of 2a, verifying compound 2 to be a threo-form vicinal diol. The ICD spectrum of 2 exhibited a negative Cotton effect at 300~350 nm, corresponding to a negative torsional angle of O—C—C—O moiety. So the absolute configuration of 2 was determined to be 1S, 2R.
Compound 3 was identified with the molecular formula of C12H20O3 from the molecular ion peak at m/z 212.1416 (calcd for C12H20O3 212.1412) in HREIMS. The 1H NMR and 13C NMR data of 3 resembled those of 1, except for the terminal double bond in 1 being saturated as an ethyl in 2. This was confirmed by the cross peaks of H-8/H-9/ H-10/H-11/H-12 in 1H-1H COSY spectrum. Detailed analysis on the HMBC and 1H-1H COSY spectra revealed the planar structure of 3 as 1-(5-pentylfuran-2-yl)-propane-1, 2-diol, dihydro-derivative of 1. Its di-O-isopro-pylidene derivative (3a) was synthesized and measured for ROESY experiment, in which H-2 and H-3 displayed correlations with the same methyl on the isopropyl group, indicating the erythro-form of 3. Subsequent Mo2(OAc)4 ICD experiment displayed the positive Cotton effect at about 350 nm, which is indicative for 1R, 2R-configuration. Compound 4 was deduced to maintain the same molecular formula and planar structure with 3 from their almost identical HRESIMS, 1D and 2D NMR data. The structural difference between 3 and 4 is only in the vicinal diol group. Consequently, the di-O-isopropylidene derivative (4a) was synthesized, and ROESY experiment was made. Different from 3a, H-2 and H-3 gave ROESY correlations to different methyls on the isopropyl moiety in the ROESY spectrum of 4a, verifying the threo configuration. In the ICD spectrum of 4, the positive Cotton effect at about 350 nm revealed the absolute stereochemistry to be 1R, 2S.
2 Conclusions
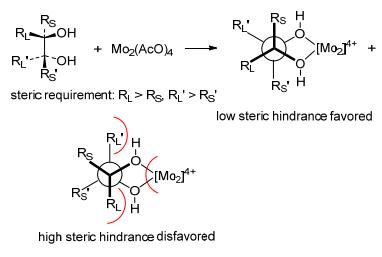
In conclusion, four closely related vicinal diols 1~4 were isolated from the cultures of the fungus Ceriporia alachuana. Their absolute configuration was totally deter-Conformer 1B was disfavored because of the increased steric hindrance of the furan moiety mined by chemical method and Mo2(OAc)4 ICD experiment. It should be noted that Mo2(OAc)4 ICD experiment is always inapplicable for non-terminal vicinal diols with a erythro-form, because the two substituents could not simultaneously be away from the Mo2(OAc)4 in Newman projection. Fortunately, the two substituents (a furan moiety and a methyl) in this case varied largely in size, and thus, the larger furan moiety stay away from the Mo2(OAc)4 to decrease the steric hindrance (Scheme 3). Eventually, the absolute configuration of the erythro-vicinal diols 1 and 3 was successfully determined. Compounds 1~4 are four unusual 2, 5-disubstituted furan derivatives with a C5-chain and a C3-diol moiety. From a biogenetic point of view, the presence of consecutive oxygenation in one terminal is interesting, and needs further investigation. This study provided valuable information for understanding the chemical diversity of Ceriporia alachuana, and determining the absolute configuration of vicinal diols.
3 Experimental
3.1 General experimental procedures
Optical rotations were recorded on a Jasco model 1020 digital polarimeter (Horiba, Tokyo, Japan). ECD spectra were performed on an Applied Photophysics Chira scan instrument (Agilent, Santa Clara, United States). Mass spectra were measured by a VG Autospec-3000 spectrometer (VG, Manchester, England). 1D and 2D NMR were recorded on AM-400 or DRX-500 spectrometer (Bruker, Bremerhaven, Germany). Silica gel (200~300 mesh, Qingdao Marine Chemical Inc., Qingdao, China), Sephadex LH-20 (Amersham Biosciences, Sweden), and RP-18 gel (40~75 μm, Fuji Silysia Chemical Ltd., Japan) were used for column chromatography. Preparative HPLC (Prep-HPLC) was performed on an Agilent 1100 liquid chromatography system equipped with a Zorbax SB-C18 column (9.4 mm×150 mm). Pre-coated silica gel GF254 plates (Qingdao Marine Chemical Inc., Qingdao, China) were used for monitoring fractions by thin-layer chromatography (TLC), and spots were visualized by heating plates after sprayed with 10% H2SO4 in ethanol.
3.2 Materials and cultivation
The fungus was collected in Beijing Botanical Garden and identified as Ceriporia alachuana by Prof. Yucheng Dai (Beijing Forestry University). A voucher specimen (BJFC005267) was deposited in the Herbarium of Beijing Forestry University. The liquid culture medium for cultivation was consist of saccharine (5%), yeast powder (0.5%), peptone (0.15%), KH2PO4 (0.05%) and MgSO4 (0.05%). C. alachuana was inoculated in a 15-literfermentor (Biostar, Shanghai Guoqiang, China) and incubated for 6 d under the following conditions: culture temperature 24 ℃, initial pH 6.0, agitation speed 250 r/min, inoculation volume 10%, and aeration rate 1.0 vvm.
3.3 Isolation
The culture broth of C. alachuana (20 L) was filtered, and the filtrate was extracted with EtOAc for three times. The combined EtOAc parts (15 g) was loaded on a silica gel column chromatography (CC) using a petroleum ether-Me2CO gradient to afford fractions A~G. Fr.E was further separated by silica gel CC using CHCl3-Me2CO system to yield three subfracions E1~E3. Fr.E2 was subjected to Sephadex LH-20 CC and eluted by MeOH to generate fractions E2-1 and E2-2. Fr.E2-2 was initially separated on an Rp-18 gel column, then purified by preparative HPLC using MeCN/H2O (V:V=40:60→70:30) system to give compounds 1 (5.0 mg), 2 (7.5 mg), 3 (20.0 mg) and 4 (21.0 mg).
(1R, 2R)-1-(5-(Pent-4-en-1-yl)furan-2-yl)propane-1, 2-di-ol (1): [α]D21-17.3 (c 0.10, CHCl3); 1H NMR (400 MHz, acetone-d6) δ: 6.16 (d, J=2.8 Hz, 1H, H-5), 5.96 (d, J=2.8 Hz, 1H, H-6), 5.88~5.78 (m, 1H, H-11), 5.02 (d, J=17.1 Hz, 1H, H-12a), 4.95 (d, J=10.2 Hz, 1H, H-12b), 4.44 (d, J=5.3 Hz, 1H, OH-3), 4.28~4.24 (m, 1H, H-3), 3.99~3.95 (m, 1H, H-2), 3.59 (OH-2), 2.59 (t, J=7.5 Hz, 2H, H-8), 2.12~2.08 (m, 2H, H-10), 1.70~1.68 (m, 2H, H-9), 1.14 (d, J=6.3 Hz, 3H, H-1); 13C NMR (100 MHz, acetone-d6) δ: 155.4 (C-4), 155.1 (C-7), 139.1 (C-11), 115.2 (C-12), 107.8 (C-5), 106.2 (C-6), 72.9 (C-3), 70.1 (C-2), 33.8 (C-10), 28.1 (C-9), 27.9 (C-8), 18.8 (C-1); HREIMS calcd for C12H18O3 210.1256, found 210.1257.
(1S, 2R)-1-(5-(Pent-4-en-1-yl)furan-2-yl)propane-1, 2-di-ol (2): [α]D21-18.3 (c 0.18, CHCl3); 1H NMR (400 MHz, acetone-d6) δ: 6.16 (d, J=2.8 Hz, 1H, H-5), 5.97 (d, J=2.8 Hz, 1H, H-6), 5.84~5.82 (m, 1H, H-11), 5.01 (d, J=17.1 Hz, 1H, H-12a), 4.94 (d, J=10.2 Hz, 1H, H-12b), 4.34 (d, J=5.3 Hz, 1H, OH-3), 4.26~4.22 (m, 1H, H-3), 3.92~3.90 (m, 1H, H-2), 3.86 (OH-2), 2.59 (t, J=7.5 Hz, 2H, H-8), 2.09~2.11 (m, 2H, H-10), 1.69~1.67 (m, 2H, H-9), 1.00 (d, J=6.3 Hz, 3H, H-1); 13C NMR (100 MHz, acetone-d6) δ: 155.8 (C-4), 154.6 (C-7), 139.1 (C-11), 115.3 (C-12), 108.2 (C-5), 106.2 (C-6), 73.4 (C-3), 70.3 (C-2), 33.7 (C-10), 28.1 (C-9), 27.8 (C-8), 19.4 (C-1); HREIMS calcd for C12H18O3 210.1256, found 210.1255.
(1R, 2R)-1-(5-Pentylfuran-2-yl)propane-1, 2-diol (3): [α]D21-17.2 (c 0.35, CHCl3); 1H NMR (500 MHz, DMSO-d6) δ: 6.08 (d, J=3.0 Hz, 1H, H-5), 5.96 (d, J=3.0 Hz, 1H, H-6), 5.15 (OH-3), 4.49 (OH-2), 4.22 (t, J=5.6 Hz, 1H, H-3), 3.78~3.74 (m, 1H, H-2), 2.53 (t, J=7.5 Hz, 2H, H-8), 1.56~1.54 (m, 2H, H-9), 1.31~1.19 (m, 4H, H-10, 11), 1.06 (d, J=6.3 Hz, 3H, H-1), 0.86 (t, J=6.8 Hz, 3H, H-12); 13C NMR (125 MHz, DMSO-d6) δ: 154.7 (C-4), 154.1 (C-7), 106.5 (C-5), 105.1 (C-6), 71.4 (C-3), 68.5 (C-2), 30.8 (C-10), 27.3 (C-8 and C-9), 21.8 (C-11), 19.0 (C-1), 13.9 (C-12); HREIMS calcd for C12H20O3 212.1412, found 212.1416.
(1R, 2S)-1-(5-Pentylfuran-2-yl)propane-1, 2-diol (4): [α]D21-2.5 (c 0.30, CHCl3); 1H NMR (400 MHz, DMSO-d6) δ: 6.11 (d, J=2.8 Hz, 1H, H-5), 5.97 (d, J=3.0 Hz, 1H, H-6), 5.15 (OH-3), 4.63 (OH-2), 4.14 (t, J=5.7 Hz, 1H, H-3), 3.75~3.71 (m, 1H, H-2), 2.54 (t, J=7.5 Hz, 2H, H-8), 1.56~1.54 (m, 2H, H-9), 1.29~1.27 (m, 4H, H-10, 11), 0.89 (d, J=6.3 Hz, 3H, H-1), 0.85 (t, J=6.7 Hz, 3H, H-12); 13C NMR (100 MHz, DMSO-d6) δ: 154.4 (C-4), 154.1 (C-7), 107.1 (C-5), 105.3 (C-6), 71.9 (C-3), 68.9 (C-2), 30.8 (C-10), 27.3 (C-8 and C-9), 21.9 (C-11), 19.2 (C-1), 13.9 (C-12); HREIMS calcd for C12H20O3 212.1412, found 212.1417.
3.4 Preparation of acetonides
Compounds 1~4 (2.0 mg) were dissolved in 2 mL DMF, respectively, then 2, 2-dimethoxypropane (2 equiv. 2.1 mg) and p-TsOH (0.5 equiv., 0.9 mg) were added. The mixture was stirred at room temperature for 3 h. The reaction was quenched by adding saturated aqueous NaHCO3 (5 mL), and extracted with EtOAc (5 mL×3). The combined organic layer was dried over Na2SO4 and concentrated in vacuo. The residue was subjected to silica gel CC using petroleum ether-Me2CO (V:V=95:5) to afford respective acetonide.
3.5 Measurements of the ICD spectra
Samples were dissolved in appropriate DMSO (spectroscopy grade), respectively, which was used for the measurement of CD spectra (CD0). Then, a quantity of Mo2(OAc)4 were added to the solution with the ligand-to-metal ratio of approximately 1:1.2. After 30 min, the CD spectra of the ligand-metal complex were further measured (CD1). The induced CD (ICD) spectra were calculated from the CD of the ligand-metal complex (CD1) deducting that of the vicinal diol sample (CD0).
Supporting Information 1D and 2D NMR of compounds 1~4. The Supporting Information is available free of charge via the Internet at http://sioc-journal.cn.
-
-
[1]
Takatsuto, S.; Ikekawa, N. J. Chem. Soc., Perkin Trans. 1 1983, 2133.
-
[2]
Thadepalli, H.; Chuah, S. K.; Iskandar, L.; Gollapudi, S. Int. J. Antimicrob. Agents 2002, 20, 180. doi: 10.1016/S0924-8579(02)00176-0
-
[3]
Nakakita, Y.; Nakagawa, M.; Sakai, H. J. Antibiot. 1980, 33, 514. doi: 10.7164/antibiotics.33.514
-
[4]
Roush, W. R.; Hartz, R. A.; Gustin, D. J. J. Am. Chem. Soc. 1999, 121, 1990. doi: 10.1021/ja984229e
-
[5]
Kolb, H. C.; Van Nieuwenhze, M. S.; Sharpless, K. B. Chem. Rev. 1994, 94, 2483. doi: 10.1021/cr00032a009
-
[6]
Harada, N.; Nakanishi, K. Acc. Chem. Res. 1972, 5, 257. doi: 10.1021/ar50056a001
-
[7]
Nakanishi, K.; Berova, N. Circular Dichroism: Principles and Applications, 2nd ed., Ed.: Woody, R. W., Weily-VCH, New York, 2000, Chapter 12.
-
[8]
Bukhari, S. T. K.; Guthrie, R. D.; Scott, A. I.; Wrixon, A. D. Chem. Commun. 1968, 24, 1580.
-
[9]
Bukhari, S. T. K.; Guthrie, R. D.; Scott, A. I.; Wrixon, A. D. Tetrahedron 1970, 26, 3653. doi: 10.1016/S0040-4020(01)92943-6
-
[10]
Scott, I.; Wrixon, A. D. Chem. Commun. 1969, 20, 1184.
-
[11]
Nakanishi, K.; Schooley, D. A.; Koreeda, M.; Dillon, J. Chem. Commun. 1971, 20, 1235.
-
[12]
Dillon, J.; Nakanishi, K. J. Am. Chem. Soc. 1974, 96, 4055. doi: 10.1021/ja00819a076
-
[13]
Dillon, J.; Nakanishi, K. J. Am. Chem. Soc. 1975, 97, 5409. doi: 10.1021/ja00852a015
-
[14]
Frelek, J.; Ikekawa, N.; Takatsuto, S.; Snatzke, G. Chirality 1997, 9, 578. doi: 10.1002/(ISSN)1520-636X
-
[15]
Bari, L. D.; Pescitelli, G.; Pratelli, C.; Pini, D.; Salvadori, P. J. Org. Chem. 2001, 66, 4819. doi: 10.1021/jo010136v
-
[16]
Xu, L. X.; He, Z. X.; Xue, J. H.; Chen, X. P.; Wei, X. Y. J. Nat. Prod. 2010, 73, 885. doi: 10.1021/np900853n
-
[17]
Liu, J.; Du, D.; Si, Y. K.; Lv, H. N.; Wu, X. F.; Li, Y.; Liu, Y. Y.; Yu, S. S. Chin. J. Org. Chem. 2010, 30, 1270.
-
[1]
-
-

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 5
- 文章访问数: 1515
- HTML全文浏览量: 143


 下载:
下载:





 下载:
下载:

