
Citation: Li Jun, Yang Tianyu, Zhang Huaiyuan, Huang Danfeng, Wang Kehu, Su Yingpeng, Hu Yulai. One-Pot Synthesis of Trifluoromethylated Homoallylic N-Acylhydrazines Promoted by Indium Powder[J]. Chinese Journal of Organic Chemistry, 2017, 37(4): 925-935. doi: 10.6023/cjoc201611009

铟粉促进下“一锅法”合成三氟甲基高烯丙基酰肼化合物
English
One-Pot Synthesis of Trifluoromethylated Homoallylic N-Acylhydrazines Promoted by Indium Powder
-
酰肼是一类很重要的有机化合物, 许多酰肼及其衍生物都具有抗病毒、抗肿瘤、抗疟疾、杀菌等生物及生理活性[1~3], 特别是含有三氟甲基的酰肼化合物已证明具有抗癌作用[4].在农业上, 酰肼化合物被广泛用作杀虫剂[5], 例如二酰肼化合物RH-5849及RH-0345已被商品化, 具有很好的杀虫能力.酰肼类化合在材料领域也表现出了潜在的应用, 可用作电致发光及液晶材料[6].在有机合成中, 酰肼常作为合成砌块, 合成各种含氮有机化合物[7].
三氟甲基是一类常见的含氟基团, 由于将其引入有机化合物分子中可以显著地改变有机化合物的极性、亲脂性以及代谢稳定性, 使得这类化合物在医药、农药和材料等领域得到了广泛的应用.因此, 如何向有机分子中引入三氟甲基成为研究热点[8].目前, 向有机分子中引入三氟甲基的方法主要有两种:一是通过三氟甲基化试剂, 利用亲核、亲电及自由基反应直接向有机分子中引入三氟甲基; 另一种是通过三氟甲基合成砌块引入三氟甲基.虽然前者在合成三氟甲基化合物中非常直接、有效, 但也有一定缺陷.例如三氟甲基化试剂比较昂贵、种类偏少、能适应的反应和底物有限等.因此利用工业上易得、廉价、稳定的含三氟甲基的有机小分子为合成砌块的方法也受到了化学家们的高度关注和研究[9].另外, 有机铟试剂是重要的有机金属化合物之一.其中, 金属铟参与的各种反应, 特别是烯丙基化反应已在有机合成中被广泛应用[10].本文报道在铟粉促进下, 以三氟乙醛甲基半缩醛或三氟甲基苯乙酮为三氟甲基合成砌块, 与酰肼、烯丙基溴“一锅法”反应可顺利得到含三氟甲基的高烯丙基酰肼化合物, 为三氟甲基高烯丙基酰肼类化合物的合成提供了一种高效简捷的新方法.
1 结果与讨论
1.1 铟粉促进下以三氟乙醛甲基半缩醛为三氟甲基合成砌块的“一锅法”反应
首先, 尝试了铟粉促进下三氟乙醛甲基半缩醛、苯甲酰肼和烯丙基溴的“一锅法”反应 (Eq. 1).结果发现, 在室温条件下反应不发生; 当升高反应温度到50 ℃和回流条件时, 分别以45%和54%的产率得到了产物4a (表 1, Entries 1~3).然后, 对该反应的物料比进行了探索 (表 1, Entries 4~10).研究表明, 当三氟乙醛甲基半缩醛 (1)、苯甲酰肼 (2a)、烯丙基溴 (3a) 和铟粉的物质的量比为1.5:1:2.5:1.5时, 4a的产率可达到83%(表 1, Entry 5).在此物料比下, 我们又考察了二氯甲烷、乙醇、甲苯、1, 4-二氧六环和水等不同溶剂对该反应的影响 (表 1, Entries 11~15).结果发现, 四氢呋喃是该反应的最佳溶剂.因此, 得到生成化合物4a的最佳反应条件:三氟乙醛甲基半缩醛 (1)、苯甲酰肼 (2a)、烯丙基溴 (3a) 和铟粉的物质的量比为1.5:1:2.5:1.5, 溶剂为四氢呋喃, 反应温度为回流.
Entry Molar ratio of 1/2a/3/In Solvent Temp./℃ Isolated yield/% 1 1.5/1/1.5/1.5 THF r.t. 0 2 1.5/1/1.5/1.5 THF 50 45 3 1.5/1/1.5/1.5 THF Reflux 54 4 1.5/1/2/1.5 THF Reflux 75 5 1.5/1/2.5/1.5 THF Reflux 83 6 1.5/1/3/1.5 THF Reflux 85 7 2/1/2.5/1.5 THF Reflux 84 8 1/1/2.5/1.5 THF Reflux 71 9 1/1.5/2.5/1.5 THF Reflux 73 10 1/2/2.5/1.5 THF Reflux 76 11 1.5/1/2.5/1.5 CH2Cl2 Reflux 77 12 1.5/1/2.5/1.5 EtOH Reflux 64 13 1.5/1/2.5/1.5 Toluene Reflux 56 14 1.5/1/2.5/1.5 1, 4-Dioxane Reflux 60 15 1.5/1/2.5/1.5 H2O Reflux 42 a A mixture of 1 (0.15~0.3 mmol), benzoylhydrazine (0.15~0.3mmol), solvent (4.0 mL) was staired at reflux for 3 h, then 3a (0.225~0.0.45 mmol), In powder (0.225 mmol) were added and stair at reflux for another 21 h. 表 1 合成化合物4a的反应条件优化a
Table 1. Optimization of reaction condition for the synthesis of compound 4a在上述最佳反应条件下, 我们用其他酰肼代替苯甲酰肼, 探索了该反应的适用范围 (Eq. 2).由表 2可知, 无论芳香酰肼还是杂环酰肼, 都能与三氟乙醛甲基半缩醛、烯丙基溴和铟粉发生“一锅法”反应, 得到相应的三氟甲基高烯丙基酰肼化合物.而且, 苯环上的取代基的电子性质对反应影响不大:不管取代基是吸电子的还是供电子的, 所得产物的产率大部分都在78%以上 (Entries 1~9).但是, 取代基在苯环上的位置对反应有较大影响.例如p-甲基苯甲酰肼作为底物反应时, 4c产率为83%, 而m-甲基苯甲酰肼和o-甲基苯甲酰肼反应时, 4d和4e的反应产率分别降为76%和56% (Entries 2~4).对于链状脂肪酰肼, 无论碳链的长短, 反应都没有发生 (Entries 10~12).
Entry R1 Product Isolated yield/% 1 p-MeOC6H4 4b 90 2 p-MeC6H4 4c 83 3 m-MeC6H4 4d 72 4 o-MeC6H4 4e 56 5 p-CF3C6H4 4f 81 6 p-FC6H4 4g 78 7 p-ClC6H4 4h 86 8 m-BrC6H4 4i 85 9 2-Furyl 4j 85 10 Me 4k 0 11 i-C3H7 4l 0 12 n-C17H35 4m 0 a A mixture of 1 (0.3 mmol), acylhydrazine (0.2 mmol) in THF (4.0 mL) was stirred at reflux for 3 h, then 3a (0.5mmol) and In powder (0.3 mmol) were added and stirred at reflux for another 21 h. 表 2 酰肼的适用范围a
Table 2. Substrate scope of acylhydrazines然后, 我们使用各种取代的烯丙基溴进一步拓展该反应的适用范围 (Eq. 3).由表 3可知, 对于γ-取代的烯丙基溴, 当取代基是供电子的甲基和较大的苯环时, 反应都能顺利进行, 并且得到的均是γ-加成产物, 其产率都在74%以上 (Entries 1~11).但是当取代基是吸电子的酯基CO2Me时, 反应却不能发生 (Entry 12).而且对于巴豆基溴 (3c), 反应表现出一定的立体选择性.其中, anti-和syn-加成产物的比率是60:40 (Entries 6~9)[11]; 肉桂基溴 (3d), 反应却没有表现出明显的立体选择性, 所得产物的anti-和syn-比率为48:52和56:44 (Entries 10~11).对于β-取代的烯丙基溴 (3f), 反应也都能顺利发生, 产物4y和4z的产率分别为81%和76%.
Entry R1 3 Product Isolated yield/% 1 C6H5 3b 4n 80 2 p-MeC6H4 3b 4o 82 3 p-MeOC6H4 3b 4p 86 4 P-FC6H4 3b 4q 74 5 p-ClC6H4 3b 4r 78 6 C6H5 3c 4s 82 b 7 p-MeC6H4 3c 4t 84 b 8 p-MeOC6H4 3c 4u 88 b 9 P-FC6H4 3c 4v 76 b 10 C6H5 3d 4w 83 c 11 p-MeOC6H4 3d 4x 86 d 12 C6H5 3e 0 13 C6H5 3f 4y 81 14 m-MeC6H4 3f 4z 76 a A mixture of 1 (0.3 mmol), acylhydrazine (0.2 mmol) in THF (4.0 mL) was stirred at reflux for 3 h, then 3 (0.5 mmol) and In powder (0.3 mmol) were added and stirred at reflux for another 21 h. b anti/syn=60/40, determined by 19F NMR analyses. c anti/syn=48/52, determined by 19F NMR analyses. d anti/syn=56/44, determined by 19F NMR analyses. 表 3 烯丙基溴的适用范围a
Table 3. Substrate scope of allyl bromides1.2 铟粉促进下以三氟苯乙酮为三氟甲基合成砌块的“一锅法”反应
在上述研究基础上, 我们将氟源改为三氟甲基苯乙酮, 尝试了铟粉促进下, 三氟甲基苯乙酮 (5a) 与苯甲酰肼 (2a) 和烯丙基溴 (3a) 的“一锅法”反应 (Eq. 4).结果发现, 在三氟乙醛甲基半缩醛作为三氟甲基合成砌块的“一锅法”反应的最优条件下, 三氟甲基苯乙酮与苯甲酰肼、烯丙基溴和铟粉的“一锅法”反应并没有发生 (表 4, Entry 1).通过薄层色谱 (TLC) 监测反应和分析该反应的混合物, 我们发现三氟甲基苯乙酮和苯甲酰肼在此反应条件下并没有生成相应的酰腙.考虑到酮的反应活性比醛的低, 我们尝试在该反应中分别加入催化量的Et2O·BF3和Ni (ClO)4·6H2O, 然而反应结果并不理想 (表 4, Entries 2~3).当用质子酸对甲苯磺酸 (PTSA) 作催化剂时, 虽然目标产物6a的产率没有显著提高 (表 4, Entry 4), 但是反应体系中有大量的5a与2a形成的酮酰腙 (6a') 生成, 并且6a'可以分离得到; 我们又在该反应体系中加入10%的Ni (ClO)4·6H2O活化酮酰腙, 促进它与烯丙基铟试剂的加成反应.令人高兴的是, 反应效果非常好, 6a的产率达到了83%(表 4, Entry 5).
 表 4
合成化合物6a的反应条件优化
Table 4.
Optimization of the reaction conditions for the synthesis of compound 6a
表 4
合成化合物6a的反应条件优化
Table 4.
Optimization of the reaction conditions for the synthesis of compound 6a
Entry Molar ratio of 5a/2a/3a/In Catalyst Isolated yield/% 1 1/1.2/2.5/1.5 None 0 2 1/1.2/2.5/1.5 Et2O·BF3 (20%) Trace 3 1/1.2/2.5/1.5 Ni (ClO)4·6H2O (20%) 12 4 1/1.2/2.5/1.5 PTSA (20%) 24 5b 1/1.2/2.5/1.5 PTSA (20%) Ni (ClO)4·6H2O (10%) 83 a A mixture of 5a (0.2 mmol), 2a (0.24 mmol), catalyst (20%, 0.04 mmol) in THF (3.0 mL) was stirred at reflux for 5 h, then 3a (0.5 mmol), In powder (0.3 mmol) were added and stirred at reflux for another 19 h. b 5a (0.2 mmol), 2a (0.24 mmol), PTSA (20%, 0.04 mmol), in THF (3.0 mL), after stirred at reflux for (5 h), then Ni (ClO)4·6H2O (10%, 0.02 mmol), 3a (0.5 mmol), In powder (0.3 mmol) were stirred at reflux for another 19 h. 表 4 合成化合物6a的反应条件优化
Table 4. Optimization of the reaction conditions for the synthesis of compound 6a因此, 在PTSA和Ni (ClO)4·6H2O的双催化下, 我们又探索了不同的三氟甲基酮和不同的酰肼与烯丙基溴和铟粉的“一锅法”反应 (Eq. 5).由表 5可知, 对于芳香酰肼和三氟甲基苯乙酮, 不管苯环上连有供电子基还是吸电子基, 反应都能顺利进行并且产率都在76%以上 (Entries 1~9).杂环的呋喃酰肼的反应产率也能到达80% (Entry 5).令人遗憾的是脂肪族酰肼, 如乙酰肼和硬脂酰肼, 仍然不能发生反应 (Entries 10~11).
Entry R1 R4 Product Isolated yield/% 1 p-MeOC6H4 Ph 6b 88 2 p-MeC6H4 Ph 6c 86 3 m-MeC6H4 Ph 6d 82 4 p-CF3C6H4 Ph 6e 80 5 p-ClC6H4 Ph 6f 85 6 2-Furyl Ph 6g 80 7 Ph m-ClC6H4 6h 82 8 Ph p-MeOC6H4 6i 88 9 Ph m-MeC6H4 6j 82 10 m-MeC6H4 m-MeC6H4 6k 83 12 Me Ph 6l 0 13 n-C17H35 Ph 6m 0 a A mixture of 5a (0.2 mmol), acylhydrazines (0.24 mmol), PTSA (20%, 0.04 mmol), in THF (3.0 mL) was stirred at reflux for 5 h, then Ni (ClO)4·6H2O (10%, 0.02 mmol), 3a (0.5 mmol) and In powder (0.3 mmol) were added and stirred at reflux for another 19 h. 表 5 三氟甲基酮和酰肼的适用范围a
Table 5. Substrate scope of trifluoromethylketones and acylhydrazines1.3 反应机理探讨
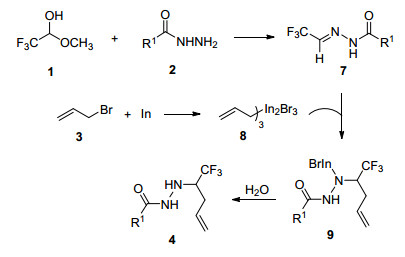
在文献报道[10a, 12]和实验基结果基础上, 我们认为铟粉促进下, “一锅法”合成三氟甲基高烯丙基酰肼化合物的机理可能如下 (Scheme 1, 以三氟乙醛甲基半缩醛为例说明):首先, 三氟乙醛甲基半缩醛1和酰肼2反应形成酰腙7, 然后7与金属铟和烯丙基溴3a形成的烯丙基铟试剂8发生亲核加成反应, 得到中间体9, 最后9水解得到4.
2 结论
综上所述, 本文报道了铟粉促进下, 以四氢呋喃为溶剂, 在回流条件下, 由三氟乙醛甲基半缩醛或三氟甲基苯乙酮、酰肼和烯丙基溴“一锅法”合成三氟甲基高烯丙基类化合物的反应.该方法以三氟乙醛甲基半缩醛或三氟甲基苯乙酮作为三氟甲基合成砌块, 具有反应原料价廉易得, 操作简单、反应条件温和和产率高等优点, 为合成三氟甲基高烯丙基类化合物提供了一种新方法.
3 实验部分
3.1 仪器与试剂
核磁共振谱用BRUKER PT jxf790425AM 400MHz型核磁共振仪测定, 以氘代氯仿作为溶剂, TMS为内标; 高分辨质谱用Bruker APEX Ⅱ傅里叶变换离子回旋共振质谱仪测定, ESI源; 熔点测定用显微熔点测定仪测定, 温度未校正.化合物2b~2m按照文献[13]方法自制, 其它试剂均为国产分析纯级.
3.2 实验方法
3.2.3 化合物6a'的合成
在50 mL的圆底烧瓶中, 依次加入三氟甲基苯乙酮 (5a) (0.034 g, 0.20 mmol)、苯甲酰肼 (2a) (0.033 g, 0.24 mmol)、对甲苯磺酸 (0.007 g, 0.04 mmol) 和THF 3 mL, 将混合物在回流条件下搅拌. TLC检测反应, 5 h后有酰腙大量的生成.然后向反应混合物中加入3 mL饱和氯化钠溶液, 搅拌5 min, 用乙酸乙酯萃取 (5 mL×3), 分出有机相, 用无水硫酸镁干燥, 蒸去溶剂后, 进行柱层析[V(石油醚):V(乙酸乙酯)=3:1]分离得N'-苯甲酰基三氟苯乙酮腙 (6a') 55.1 mg, 产率94%.白色固体, m.p. 143~144 ℃; 1H NMR (400 MHz, CDCl3) δ: 9.08 (s, 1H), 7.71 (s, 1H), 7.60 (d, J=1.2 Hz, 3H), 7.54 (t, J=7.6 Hz, 1H), 7.42~7.05 (m, 4H); 13C NMR (150 MHz, CD3CO-CD3) δ: 165.8, 141.4, 133.9, 133.1, 132.2, 130.8, 129.6, 129.3, 127.6, 122.1 (q, JC—F=271.5 Hz), 29.96 (dp, JC—F=38.5, 19.5 Hz); 19F NMR (376 MHz, CDCl3)δ: -68.4 (s); HRMS (ESI) calcd for C15H12F3N2O[M+H]+ 293.0896, found 293.0894.
辅助材料 (Supporting Information) 合成产物的核磁共振氢谱、碳谱和氟谱以及高分辨质谱.这些材料可以免费从本刊网站 (http://sioc-journal.cn/) 上下载.
3.2.1 化合物4的合成
在50 mL的圆底烧瓶中, 依次加入三氟乙醛甲基半缩醛 (1) (0.039 g, 0.30 mmol)、苯甲酰肼 (2a) (0.027 g, 0.20 mmol) 和4 mL四氢呋喃 (THF), 将此混合液在回流条件下搅拌. TLC检测反应, 3 h后有酰腙大量生成.此时加入烯丙基溴 (3a) (0.060 g, 0.50 mmol) 和铟粉 (0.034 g, 0.30 mmol), 继续回流此反应混合物, TLC检测21 h反应完全.然后向反应混合物中加入3 mL饱和氯化铵溶液, 搅拌10 min, 用乙酸乙酯萃取 (5 mL×3), 分出有机相, 用无水硫酸镁干燥, 蒸去溶剂后, 进行柱层析[V(石油醚):V(乙酸乙酯)=5:1]分离得N'-(1-三氟甲基丁-3-烯-1-基) 苯甲酰肼 (4a) 42.08 mg, 产率83%.白色固体, m.p. 96~97 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.74~7.72 (m, 3H), 7.55 (s, 1H), 7.46 (d, J=6.8 Hz, 2H), 6.02~6.00 (m, 1H), 5.33 (d, J=14.8 Hz, 2H), 4.94 (s, 1H), 3.50 (s, 1H), 2.64~2.61 (m, 1H), 2.39~2.31 (m, 1H); 13C NMR (100 MHz, CDCl3) δ: 167.3, 132.1, 132.0, 131.91, 128.63, 126.79, 125.9 (q, JC—F=279.4 Hz), 119.9, 60.5 (q, JC—F=26.8 Hz), 31.4 (d, JC—F=2.1 Hz); 19F NMR (376 MHz, CDCl3) δ: -75.5 (d, JF—H=6.8 Hz); HRMS (ESI) calcd for C12H14F3N2O[M+H]+ 259.1053, found 259.1051.
化合物4b~4z的合成方法同4a.
N'-(1-三氟甲基丁-3-烯-1-基)-4-甲氧基苯甲酰肼 (4b): 51.8 mg, 产率90%.白色固体, m.p. 99~100 ℃; 1H NMR (600 MHz, CDCl3) δ: 7.73 (s, 1H), 7.69 (d, J=8.4 Hz, 2H), 6.91 (d, J=9.0 Hz, 2H), 6.02~5.95 (m, 1H), 5.31~5.28 (m, 2H), 4.92 (d, J=6.0 Hz, 1H), 3.84 (s, 3H), 3.49~3.44 (m, 1H), 2.61~2.58 (m, 1H), 2.36~2.30 (m, 1H); 13C NMR (150 MHz, CDCl3) δ: 167.0, 162.7, 132.1, 128.7, 126.1 (q, JC-F=279.6 Hz), 124.4, 119.9, 113.9, 60.7 (q, JC—F=26.7 Hz), 55.4, 31.5 (d, JC—F=2.1 Hz); 19F NMR (376 MHz, CDCl3)δ: -75.5 (d, JF—H=6.8 Hz); HRMS (ESI) calcd for C13H16F3N2O2 [M+H]+ 289.1158, found 289.1157.
N'-(1-三氟甲基丁-3-烯-1-基)-4-甲基苯甲酰肼 (4c): 45.1 mg, 产率83%.白色固体, m.p. 100~101 ℃; 1H NMR (600 MHz, CDCl3) δ: 7.81 (br, 1H), 7.62 (d, J=7.8 Hz, 2H), 7.23 (d, J=7.8 Hz, 2H), 6.02~5.95 (m, 1H), 5.31~5.28 (m, 2H), 4.92 (d, J=5.4 Hz, 1H), 3.47 (s, 1H), 2.59 (d, J=15.0 Hz, 1H), 2.39 (s, 3H), 2.36~2.30 (m, 1H); 13C NMR (150 MHz, CDCl3) δ: 167.4, 142.8, 132.0, 129.4, 129.3, 126.9, 126.0 (q, JC—F=279.4 Hz), 119.9, 60.6 (q, JC—F=26.7 Hz), 31.5 (d, JC—F=2.1 Hz), 21.5; 19F NMR (376 MHz, CDCl3) δ: -75.5 (d, JF—H=6.8 Hz); HRMS (ESI) calcd for C13H16F3N2O[M+H]+ 273.1209, found 273.1214.
N'-(1-三氟甲基丁-3-烯-1-基)-3-甲基苯甲酰肼 (4d): 39.1 mg, 产率72%.白色固体, m.p. 75~77 ℃; 1H NMR (600 MHz, CDCl3) δ: 7.82 (d, J=6.0 Hz, 1H), 7.54 (s, 1H), 7.49 (d, J=7.2 Hz, 1H), 7.33 (d, J=7.2 Hz, 1H), 7.32~7.30 (m, 1H), 6.02~5.95 (m, 1H), 5.32~5.28 (m, 2H), 4.92 (d, J=6.0 Hz, 1H), 3.51~3.45 (m, 1H), 2.60 (d, J=14.4 Hz, 1H), 2.38 (s, 3H), 2.36~2.31 (m, 1H); 13C NMR (150 MHz, CDCl3) δ: 167.6, 138.6, 132.9, 132.1, 132.1, 128.6, 127.6, 126.1 (q, JC—F=279.6 Hz), 123.8, 119.9, 60.6 (q, JC—F=26.7 Hz), 31.5 (d, JC—F=2.1 Hz), 21.3; 19F NMR (376 MHz, CDCl3) δ: -75.5 (d, JF—H=6.8 Hz); HRMS (ESI) calcd for C13H16F3N2O[M+H]+ 273.1209, found 273.1215.
N'-(1-三氟甲基丁-3-烯-1-基)-2-甲基苯甲酰肼 (4e): 30.5 mg, 产率56%.白色固体, m.p. 79~80 ℃; 1H NMR (600 MHz, CDCl3) δ: 7.42 (s, 1H), 7.36~7.31 (m, 2H), 7.23~7.18 (m, 2H), 6.06~5.95 (m, 1H), 5.35~5.30 (m, 2H), 4.91 (d, J=6.4 Hz, 1H), 3.57~3.48 (m, 1H), 2.65~2.59 (m, 1H), 2.43 (s, 3H), 2.39~2.31 (m, 1H); 13C NMR (150 MHz, CDCl3) δ: 169.4, 136.8, 133.3, 131.9, 131.2, 130.6, 127.0, 126.0 (q, JC—F=279.6 Hz), 125.8, 120.1, 60.6 (q, J=26.7 Hz), 31.5 (d, JC—F=2.1 Hz), 19.6; 19F NMR (376 MHz, CDCl3) δ: -75.3 (d, JF—H=6.8 Hz, ); HRMS (ESI) calcd for C13H16F3N2O[M+H]+ 273.1209, found 273.1216.
N'-(1-三氟甲基丁-3-烯-1-基)-4-三氟甲基苯甲酰肼 (4f): 52.8 mg, 产率81%.白色固体, m.p. 87~89 ℃; 1H NMR (600 MHz, CDCl3) δ: 7.95 (s, 1H), 7.83 (d, J=7.8 Hz, 2H), 7.70 (d, J=8.4 Hz, 2H), 6.01~5.94 (m, 1H), 5.33~5.29 (m, 2H), 4.93 (d, J=6.0 Hz, 1H), 3.53~3.47 (m, 1H), 2.63~2.60 (m, 1H), 2.36~2.28 (m, 1H); 13C NMR (150 MHz, CDCl3) δ: 166.2, 135.4, 133.9 (q, JC—F=32.5 Hz), 131.8, 127.4, 126.0 (q, JC—F=279.6 Hz), 125.8 (d, JC—F=3.5 Hz), 123.4 (q, JC—F=271.2 Hz), 120.3, 60.5 (q, JC—F=27.1 Hz), 31.4 (d, JC—F=2.1 Hz); 19F NMR (376 MHz, CDCl3) δ: -63.6 (d, JF-H=5.3 Hz), -75.5 (d, JF—H=6.8 Hz); HRMS (ESI) calcd for C13H13F6N2O[M+H]+ 327.0927, found 327.0926.
N'-(1-三氟甲基丁-3-烯-1-基)-4-氟苯甲酰肼 (4g): 43.0 mg, 产率78%.白色固体, m.p. 81~82 ℃; 1H NMR (600 MHz, CDCl3) δ: 7.75 (s, 1H), 7.74~7.73 (m, 2H), 7.12 (t, J=8.4 Hz, 2H), 6.01~5.96 (m, 1H), 5.32~5.30 (m, 2H), 4.91 (d, J=6.0 Hz, 1H), 3.50~3.45 (m, 1H), 2.61 (d, J=14.4 Hz, 1H), 2.36~2.30 (m, 1H); 13C NMR (150 MHz, CDCl3) δ: 166.4, 165.1 (d, JC—F=251.9 Hz), 131.9, 129.3 (d, JC—F=9.0 Hz), 128.3 (d, JC—F=3.2 Hz), 126.0 (q, JC—F=279.6 Hz), 120.1, 115.9 (d, JC—F=22.0 Hz), 60.6 (q, JC—F=26.5 Hz), 31.5 (d, JC—F=2.1 Hz); 19F NMR (376 MHz, CDCl3) δ: -75.5 (d, JF—H=6.8 Hz), -107.1 (m); HRMS (ESI) calcd for C12H12NaF4N2O[M+Na]+ 299.0778, found 299.0783.
N'-(1-三氟甲基丁-3-烯-1-基)-4-氯苯甲酰肼 (4h): 50.2 mg, 产率86%.白色固体, m.p. 114~115 ℃; 1H NMR (600 MHz, CDCl3) δ: 7.81 (s, 1H), 7.67 (d, J=12.0 Hz, 2H), 7.42 (d, J=12.0 Hz, 2H), 6.01~5.95 (m, 1H), 5.33~5.30 (m, 2H), 4.92 (d, J=6.0 Hz, 1H), 3.51~3.46 (m, 1H), 2.63~2.59 (m, 1H), 2.36~2.31 (m, 1H); 13C NMR (150 MHz, CDCl3) δ: 166.4, 138.6, 131.9, 130.5, 129.1, 128.3, 126.0 (q, JC—F=279.5 Hz), 120.1, 60.6 (q, JC—F=26.5 Hz), 31.5 (d, JC—F=2.1 Hz); 19F NMR (376 MHz, CDCl3) δ: -75.5 (d, JF—H=6.8 Hz); HRMS (ESI) calcd for C12H13ClF3N2O[M+H]+293.0663, found 293.0662.
N'-(1-三氟甲基丁-3-烯-1-基)-3-溴苯甲酰肼 (4i): 57.1 mg, 产率85%.白色固体, m.p. 80~82 ℃; 1H NMR (600 MHz, CDCl3) δ: 7.88~7.85 (m, 2H), 7.67~7.63 (m, 2H), 7.33~7.31 (m, 1H), 6.01~5.95 (m, 1H), 5.33~5.30 (m, 2H), 4.91 (d, J=6.0 Hz, 1H), 3.51~3.46 (m, 1H), 2.64~2.60 (m, 1H), 2.37~2.31 (m, 1H); 13C NMR (150 MHz, CDCl3) δ: 166.0, 135.2, 134.1, 131.9, 130.3, 130.2, 126.0 (q, JC—F=279.6 Hz), 125.4, 122.9, 120.2, 60.6 (q, JC—F=27.0 Hz), 31.5 (d, JC—F=2.1 Hz); 19F NMR (376 MHz, CDCl3) δ: -75.4 (d, JF—H=6.8 Hz); HRMS (ESI) calcd for C12H13BrF3N2O[M+H]+ 337.0158, found 337.0159.
N'-(1-三氟甲基丁-3-烯-1-基)-2-呋喃甲酰肼 (4j): 42.2 mg, 产率85%.白色固体, m.p. 65~67 ℃; 1H NMR (600 MHz, CDCl3) δ: 7.92 (s, 1H), 7.46 (s, 1H), 7.15 (d, J=3.6 Hz, 1H), 6.52 (t, J=1.8 Hz, 1H), 6.02~5.96 (m, 1H), 5.33~5.29 (m, 2H), 4.82 (d, J=6.0 Hz, 1H), 3.49~3.47 (m, 1H), 2.62~2.59 (m, 1H), 2.36~2.31 (m, 1H); 13C NMR (150 MHz, CDCl3) δ: 158.1, 146.2, 144.6, 132.0, 126.0 (q, JC—F=279.6 Hz), 120.1, 115.3, 112.1, 60.8 (q, JC—F=27.0 Hz), 31.4 (d, JC-F=2.1 Hz); 19F NMR (376 MHz, CDCl3) δ: -75.7 (d, JF—H=6.8 Hz); HRMS (ESI) calcd for C10H12F3N2O2[M+H]+ 249.0845, found 249.0842.
N'-(1-三氟甲基-2, 2-二甲基丁-3-烯-1-基) 苯甲酰肼 (4n): 45.8 mg, 产率80%.无色粘稠油状物. 1H NMR (600 MHz, CDCl3) δ: 7.71~7.70 (m, 2H), 7.69 (d, J=1.2 Hz, 1H), 7.52~7.50 (m, 1H), 7.43~7.40 (m, 2H), 6.08 (dd, J=17.4, 10.8 Hz, 1H), 5.22~5.18 (m, 2H), 5.00 (d, J=6.0 Hz, 1H), 3.21 (q, J=8.4 Hz, 1H), 1.31 (s, 3H), 1.24 (s, 3H); 13C NMR (150 MHz, CDCl3) δ: 167.0, 143.7, 132.3, 132.1, 128.7, 126.8, 126.7 (q, JC—F=282.4 Hz), 114.0, 68.3 (q, JC—F=24.2 Hz), 39.0, 25.8, 22.4; 19F NMR (376 MHz, CDCl3) δ: -66.9 (d, JF—H=7.8 Hz); HRMS (ESI) calcd for C14H18F3N2O[M+H]+ 287.1366, found 287.1363
N'-(1-三氟甲基-2, 2-二甲基丁-3-烯-1-基)-4-甲基苯甲酰肼 (4o): 49.2 mg, 产率82%.白色固体, m.p. 129~130 ℃; 1H NMR (600 MHz, CDCl3) δ: 7.64 (d, J=6.0 Hz, 1H), 7.61 (d, J=8.4 Hz, 2H), 7.23 (d, J=8.4 Hz, 2H), 6.10 (dd, J=17.4, 10.8 Hz, 1H), 5.24~5.20 (m, 2H), 5.01 (d, J=6.0 Hz, 1H), 3.21 (q, J=8.4 Hz, 1H), 2.39 (s, 3H), 1.33 (s, 3H), 1.26 (s, 3H); 13C NMR (150 MHz, CDCl3) δ: 166.9, 143.7, 142.6, 129.5, 129.4, 126.8, 126.7 (q, JC—F=282.6 Hz), 113.9, 68.3 (q, JC—F=24.0 Hz), 39.0, 25.8 (d, JC—F=1.7 Hz), 22.5, 21.4; 19F NMR (376 MHz, CDCl3) δ: -66.9 (d, JF—H=7.8 Hz); HRMS (ESI) calcd for C15H20F3N2O[M+H]+ 301.1522, found 301.1521.
N'-(1-三氟甲基-2, 2-二甲基丁-3-烯-1-基)-4-甲氧基苯甲酰肼 (4p): 54.4 mg, 产率86%.白色固体, m.p. 80~82 ℃; 1H NMR (600 MHz, CDCl3) δ: 7.67 (d, J=8.4 Hz, 2H), 7.55 (d, J=6.0 Hz, 1H), 6.92 (d, J=8.4 Hz, 2H), 6.09 (dd, J=17.4, 10.8 Hz, 1H), 5.23~5.19 (m, 2H), 5.01 (d, J=6.0 Hz, 1H), 3.84 (s, 3H), 3.20 (q, J=7.8 Hz, 1H), 1.32 (s, 3H), 1.25 (s, 3H); 13C NMR (150 MHz, CDCl3)δ: 166.6, 162.6, 143.7, 128.7, 126.8 (q, JC—F=282.7 Hz), 124.5, 114.0, 113.9, 68.4 (q, JC—F=24.0 Hz), 55.4, 39.0, 25.8, 22.5;19F NMR (376 MHz, CDCl3) δ:-66.9 (d, JF—H=7.9 Hz); HRMS (ESI) calcd for C15H20F3N2O2[M+H]+ 317.1471, found 317.1479.
N'-(1-三氟甲基-2, 2-二甲基丁-3-烯-1-基)-4-氟苯甲酰肼 (4q): 44.9 mg, 产率74%.白色固体, m.p. 85~86 ℃; 1H NMR (600 MHz, CDCl3) δ: 7.74~7.73 (m, 2H), 7.72 (s, 1H), 7.13~7.10 (m, 2H), 6.08 (dd, J=17.4, 10.8 Hz, 1H), 5.23~5.19 (m, 2H), 5.00 (d, J=6.0 Hz, 1H), 3.22 (q, J=7.8 Hz, 1H), 1.32 (s, 3H), 1.25 (s, 3H); 13C NMR (150 MHz, CDCl3) δ: 166.0, 165.1 (d, JC—F=236.7 Hz), 143.6, 129.2 (d, JC—F=9.0 Hz), 128.5 (d, JC-F=3.0 Hz), 126.7 (q, JC—F=282.6 Hz), 115.9 (d, JC—F=21.9 Hz), 114.0, 68.3 (q, JC—F=24.4 Hz), 39.0, 25.8, 22.4; 19F NMR (376 MHz, CDCl3) δ: -66.9 (d, JF—H=7.8 Hz), -107.2 (m); HRMS (ESI) calcd for C14H16NaF4N2O[M+Na]+ 327.1091, found 327.1096.
N'-(1-三氟甲基-2, 2-二甲基丁-3-烯-1-基)-4-氯苯甲酰肼 (4r): 50.0 mg, 产率78%.白色固体, m.p. 103~105 ℃; 1H NMR (400 MHz, CDCl3) δ: 7.74 (d, J=6.0 Hz, 1H), 7.65 (d, J=8.4 Hz, 2H), 7.41 (d, J=8.0 Hz, 2H), 6.07 (dd, J=17.2, 10.8 Hz, 1H), 5.24~5.19 (m, 2H), 4.99 (d, J=6.0 Hz, 1H), 3.21 (q, J=8.0 Hz, 1H), 1.32 (s, 3H), 1.25 (s, 3H); 13C NMR (150 MHz, CDCl3) δ: 166.0, 143.6, 138.4, 130.7, 129.0, 128.3, 126.7 (q, JC—F=282.6 Hz), 114.1, 68.2 (q, JC—F=24.3 Hz), 39.0, 25.8, 22.3; 19F NMR (376 MHz, CDCl3) δ: -66.9 (d, JF—H=7.8 Hz); HRMS (ESI) calcd for C14H17ClF3N2O[M+H]+ 321.0976, found 321.0975.
N'-(1-三氟甲基-2-甲基丁-3-烯-1-基) 苯甲酰肼 (4s-anti): 44.6 mg, 产率82%.无色粘稠油状物. 1H NMR (400 MHz, CDCl3) δ: 8.26 (s, 1H), 7.63~7.61 (m, 2H), 7.37 (t, J=7.6 Hz, 1H), 7.27 (t, J=7.6 Hz, 2H), 5.92~5.82 (m, 1H), 5.11~5.03 (m, 2H), 4.84 (t, J=7.2 Hz, 1H), 3.35~3.27 (m, 1H), 2.69~2.64 (m, 1H), 1.10 (d, J=6.8 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ: 167.6, 138.6, 131.9, 128.5, 126.9, 126.1 (q, JC—F=282.0 Hz), 117.3, 116.2, 64.5 (q, JC—F=25.5 Hz), 36.5, 14.3; 19F NMR (376 MHz, CDCl3) δ: -70.4 (d, JF—H=7.5 Hz), HRMS (ESI) calcd for C13H16F3N2O[M+H]+ 273.1212, found 273.1209.
N'-(1-三氟甲基-2-甲基丁-3-烯-1-基) 苯甲酰肼 (4s-syn): 44.6 mg, 产率82%.无色粘稠油状物. 1H NMR (400 MHz, CDCl3) δ: 8.26 (s, 1H), 7.63~7.61 (m, 2H), 7.37 (t, J=7.6 Hz, 1H), 7.27 (t, J=7.6 Hz, 2H), 5.92~5.82 (m, 1H), 5.11~5.03 (m, 2H), 4.84 (t, J=7.2 Hz, 1H), 3.18~3.10 (m, 1H), 2.51~2.45 (m, 1H), 1.13 (d, J=6.8 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ: 167.4, 138.3, 132.2, 128.5, 126.8, 126.3 (q, JC—F=280.5 Hz), 117.3, 116.2, 64.9 (q, JC—F=25.5 Hz), 37.0, 17.3; 19F NMR (376 MHz, CDCl3) δ: -71.6 (d, JF—H=7.5 Hz); HRMS (ESI) calcd for C13H16F3N2O[M+H]+273.1212, found 273.1209.
N'-(1-三氟甲基-2-甲基丁-3-烯-1-基)-4-甲基苯甲酰肼 (4t-anti): 48.1 mg, 产率84%.无色粘稠油状物.1H NMR (600 MHz, CDCl3) δ: 7.95 (br, 1H), 7.54~7.52 (m, 2H), 7.12~7.11 (m, 2H), 5.94~5.89 (m, 1H), 5.14~5.08 (m, 2H), 4.83 (d, J=5.4 Hz, 1H), 3.33~3.31 (m, 1H), 2.69 (s, 1H), 2.29 (s, 3H), 1.13 (d, J=6.6 Hz, 3H); 13C NMR (150 MHz, CDCl3)δ: 167.5, 142.5, 138.6, 129.2, 126.9, 126.2 (q, JC—F=281.4 Hz), 117.4, 116.2, 64.7 (q, JC—F=25.2 Hz), 36.6, 21.3, 14.4; 19F NMR (376 MHz, CDCl3)δ: -70.4 (d, JF—H=7.5 Hz). HRMS (ESI) calcd for C14H18F3N2O[M+H]+ 287.1367, found 287.1366.
N'-(1-三氟甲基-2-甲基丁-3-烯-1-基)-4-甲基苯甲酰肼 (4t-syn): 48.1 mg, 产率84%.无色粘稠油状物. 1H NMR (600 MHz, CDCl3) δ: 7.95 (br, 1H), 7.54~7.52 (m, 2H), 7.12~7.11 (m, 2H), 5.94~5.89 (m, 1H), 5.14~5.08 (m, 2H), 4.86 (d, J=6.0 Hz, 1H), 3.17~3.12 (m, 1H), 2.53~2.49 (m, 1H), 2.29 (s, 3H), 1.16 (d, J=7.2 Hz, 3H); 13C NMR (150 MHz, CDCl3)δ: 167.3, 142.5, 138.3, 129.2, 126.8, 126.3 (q, JC—F=282.9 Hz), 117.4, 116.2, 65.1 (q, JC—F=25.2 Hz), 37.1, 21.3, 17.4; 19F NMR (376 MHz, CDCl3)δ: -71.6 (d, JF—H=7.5 Hz). HRMS (ESI) calcd for C14H18F3N2O[M+H]+287.1367, found 287.1366.
N'-(1-三氟甲基-2-甲基丁-3-烯-1-基)-4-甲氧基苯甲酰肼 (4u-anti): 53.2 mg, 产率88%.无色粘稠油状物. 1H NMR (600 MHz, CDCl3) δ: 8.17 (br, 1H), 7.60~7.58 (m, 2H), 6.78~6.74 (m, 2H), 5.90~5.83 (m, 1H), 5.10~5.02 (m, 2H), 4.83 (s, 1H), 3.70~3.68 (s, 3H), 3.29 (s, 1H), 2.66 (s, 1H), 1.09 (d, J=6.6 Hz, 3H); 13C NMR (150 MHz, CDCl3)δ: 167.2, 162.4, 138.6, 128.8, 126.2 (q, JC—F=282.0 Hz), 124.4, 116.1, 113.7, 64.5 (q, JC—F=25.5 Hz), 55.1, 36.5, 14.3; 19F NMR (376 MHz, CDCl3) δ: -70.4 (d, JF—H=7.5 Hz). HRMS (ESI) calcd for C14H18F3N2O2[M+H]+ 303.1317, found 303.1315.
N'-(1-三氟甲基-2-甲基丁-3-烯-1-基)-4-甲氧基苯甲酰肼 (4u-syn): 53.2 mg, 产率88%.无色粘稠油状物. 1H NMR (600 MHz, CDCl3) δ: 8.17 (br, 1H), 7.60~7.58 (m, 2H), 6.78~6.74 (m, 2H), 5.90~5.83 (m, 1H), 5.10~5.02 (m, 2H), 4.83 (s, 1H), 3.70~3.68 (s, 3H), 3.14~3.11(m, 1H), 2.49~2.47 (m, 1H), 1.12 (d, J=8.4 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ: 167.0, 162.4, 138.3, 128.7, 126.3 (q, JC—F=280.5 Hz), 124.4, 117.2, 113.7, 64.9 (q, JC—F=25.5 Hz), 55.1, 36.9, 17.2; 19F NMR (376 MHz, CDCl3)δ: -71.6 (d, JF—H=7.5 Hz). HRMS (ESI) calcd for C14H18F3N2O2[M+H]+ 303.1317, found 303.1315.
N'-(1-三氟甲基丁-2-甲基丁-3-烯-1-基)-4-氟苯甲酰肼 (4v-anti): 44.1 mg, 产率76%.无色粘稠油状物. 1H NMR (600 MHz, CDCl3) δ: 8.16 (br, 1H), 7.67~7.64 (m, 2H), 6.99~6.97 (m, 2H), 5.92~5.84 (m, 1H), 5.13~5.10 (m, 2H), 4.83 (d, J=4.8 Hz, 1H), 3.32 (s, 1H), 2.68 (s, 1H), 1.12 (d, J=3.6 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ: 166.7, 165.0 (d, JC—F=250.5 Hz), 138.5, 129.4 (d, JC—F=7.5 Hz), 128.4 (d, JC—F=7.5 Hz), 126.2 (q, JC—F=280.5 Hz), 116.3, 115.7 (d, JC—F=22.5 Hz), 64.6 (q, JC—F=24.0 Hz), 36.5, 14.3; 19F NMR (376 MHz, CDCl3)δ: -70.4 (d, JF—H=7.6 Hz), -107.4 (s). HRMS (ESI) calcd for C13H15F4N2O[M+H]+291.1118, found 291.1115.
N'-(1-三氟甲基丁-2-甲基丁-3-烯-1-基)-4-氟苯甲酰肼 (4v-syn): 44.1 mg, 产率76%.无色粘稠油状物. 1H NMR (600 MHz, CDCl3) δ: 8.16 (br, 1H), 7.67~7.64 (m, 2H), 6.99~6.97 (m, 2H), 5.92~5.84 (m, 1H), 5.08~5.05 (m, 2H), 4.85 (d, J=3.6 Hz, 1H), 3.17~3.12 (m, 1H), 2.54~2.46 (m, 1H), 1.15 (d, J=3.0 Hz, 3H); 13C NMR (150 MHz, CDCl3) δ: 166.5, 165.0 (d, JC—F=250.5 Hz), 138.2, 129.3 (d, JC—F=9.0 Hz), 128.5 (d, JC—F=7.5 Hz), 126.3 (q, JC—F=280.5 Hz), 117.5, 115.7 (d, JC—F=22.5 Hz), 65.0 (q, JC—F=25.5 Hz), 37.1, 17.3; 19F NMR (376 MHz, CDCl3)δ: -71.6 (d, JF—H=7.2 Hz), -107.4 (s). HRMS (ESI) calcd for C13H15F4N2O[M+H]+ 291.1118, found 291.1115.
N'-(1-三氟甲基丁-2-苯基-3-烯-1-基) 苯甲酰肼 (4w-anti): 55.5 mg, 产率83%.无色粘稠油状物. 1H NMR (600 MHz, CDCl3) δ: 7.96~7.88 (m, 1H), 7.77 (d, J=8.4 Hz, 1H), 7.70 (d, J=7.2 Hz, 1H), 7.62~7.49 (m, 2H), 7.46~7.20 (m, 6H), 6.26~6.20 (m, 1H), 5.30~5.26 (m, 2H), 5.13 (d, J=10.2 Hz, 1H), 3.98~3.93 (m, 1H), 3.78 (t, J=8.4 Hz, 1H); 13C NMR (150 MHz, CD3OD) δ: 169.1, 139.8, 138.4, 133.7, 132.7, 129.7, 129.5, 128.8, 128.3, 127.9, 126.7 (q, JC—F=279.0 Hz), 117.2, 65.15 (q, JC—F=25.5 Hz), 50.74;19F NMR (376 MHz, CD3OD) δ: -71.0 (d, J=7.1 Hz). HRMS (ESI) calcd for C18H17F3N2-ONa [M+Na]+ 335.1361, found 357.1185.
N'-(1-三氟甲基丁-2-苯基-3-烯-1-基) 苯甲酰肼 (4w-syn): 55.5 mg, 产率83%.无色粘稠油状物. 1H NMR (600 MHz, CD3OD) δ: 7.96~7.88 (m, 1H), 7.77 (d, J=8.4 Hz, 1H), 7.70 (d, J=7.2 Hz, 1H), 7.62~7.49 (m, 2H), 7.46~7.20 (m, 6H), 6.43~6.37 (m, 1H), 5.30~5.26 (m, 2H), 5.18 (d, J=17.4 Hz, 1H), 3.89~3.84 (m, 1H), 3.66 (t, J=9.0 Hz, 1H); 13C NMR (150 MHz, CD3OD) δ: 169.5, 141.4, 138.2, 133.7, 132.8, 129.6, 129.4, 129.3, 128.2, 128.1, 126.7 (q, JC—F=279.0 Hz), 118.9, 65.15 (q, JC—F=25.5 Hz), 50.74; 19F NMR (376 MHz, CD3OD) δ: -72.3 (d, J=6.7 Hz). HRMS (ESI) calcd for C18H17F3N2-ONa [M+Na]+ 335.1361, found 357.1185.
N'-(1-三氟甲基丁-2-苯基-3-烯-1-基)-4-甲氧基苯甲酰肼 (4x-anti)): 62.7 mg, 产率86%.无色粘稠油状物. 1H NMR (600 MHz, CD3OD) δ: 7.90~7.84 (m, 1H), 7.75 (d, J=8.4 Hz, 1H), 7.68 (d, J=9.0 Hz, 1H), 7.38~7.18 (m, 5H), 7.00~6.91 (m, 2H), 6.24~6.18 (m, 1H), 5.24~5.29 (m, 2H), 5.16 (d, J=16.8 Hz, 1H), 3.94~3.89 (m, 1H), 3.78 (s, 3H), 3.64 (t, J=9.0 Hz, 1H); 13C NMR (150 MHz, CD3OD) δ: 168.7, 163.8, 139.7, 138.3, 129.9, 129.5, 129.4, 128.2, 127.2 (q, JC—F=279.0 Hz), 125.6, 117.2, 114.5, 65.2 (q, JC—F=25.5 Hz), 55.6, 50.7 (d, JC—F=3.0 Hz); 19F NMR (376 MHz, CD3OD) δ: -70.9 (d, J=6.9 Hz), HRMS (ESI) calcd for C19H19F3N2O2Na[M+Na]+ 365.1468, found 387.1291.
N'-(1-三氟甲基丁-2-苯基-3-烯-1-基)-4-甲氧基苯甲酰肼 (4x-syn): 62.7 mg, 产率86%.无色粘稠油状物. 1H NMR (600 MHz, CD3OD) δ: 7.90~7.84 (m, 1H), 7.75 (d, J=8.4 Hz, 1H), 7.68 (d, J=9.0 Hz, 1H), 7.38~7.18 (m, 5H), 7.00~6.91 (m, 2H), 6.36~6.42 (m, 1H), 5.24~5.29 (m, 2H), 5.11 (d, J=10.2 Hz, 1H), 3.84~3.83 (m, 1H), 3.79 (s, 3H), 3.77~3.75 (m, 1H); 13C NMR (150 MHz, CD3OD)δ: 169.1, 163.9, 141.3, 138.2, 130.0, 129.6, 128.8, 127.8, 127.2 (q, JC—F=279.0 Hz), 125.6, 118.9, 114.6, 65.2 (q, JC—F=25.5 Hz), 55.7, 50.7 (d, JC—F=3.0 Hz); 19F NMR (376 MHz, CD3OD) δ: -72.1 (d, J=6.6 Hz). HRMS (ESI) calcd for C19H19F3N2O2Na[M+Na]+ 365.1468, found 387.1291.
N'-(1-三氟甲基丁-3-甲基-3-烯-1-基) 苯甲酰肼 (4y): 44.1 mg, 产率81%.无色粘稠油状物. 1H NMR (600 MHz, CDCl3) δ: 7.82 (br, 1H), 7.64~7.61 (m, 2H), 7.44~7.41 (m, 1H), 7.35~7.32 (m, 2H), 4.96 (s, 1H), 4.91~4.89 (m, 2H), 3.55~3.51 (m, 1H), 2.35 (d, J=14.4 Hz, 1H), 2.26~2.20 (m, 1H), 1.83 (s, 3H); 13C NMR (150 MHz, CDCl3) δ: 167.4, 139.6, 132.3, 132.1, 128.7, 126.9, 126.2 (q, JC—F=279.0 Hz), 116.11, 58.2 (q, JC—F=27.0 Hz), 35.6 (d, JC—F=1.5 Hz), 21.2; 19F NMR (376 MHz, CDCl3)δ: -75.3 (d, J=6.6 Hz). HRMS (ESI) calcd for C13H15F3N2ONa[M+Na]+ 273.1208, found 295.1029.
N'-(1-三氟甲基丁-3-甲基-3-烯-1-基)-3-甲基苯甲酰肼 (4z): 43.5 mg, 产率76%.无色粘稠油状物. 1H NMR (600 MHz, CDCl3) δ: 7.83 (br, 1H), 7.54 (s, 1H), 7.47 (d, J=7.2 Hz, 1H), 7.32~7.28 (m, 2H), 5.04 (s, 1H), 4.97 (s, 2H), 3.62~3.59 (m, 1H), 2.43 (d, J=14.4 Hz, 1H), 2.36 (s, 3H), 2.33~2.29 (m, 1H), 1.91 (s, 3H); 13C NMR (150 MHz, CDCl3)δ: 167.6, 139.6, 138.6, 132.8, 132.2, 128.5, 127.6, 126.2 (q, JC—F=279.0 Hz), 123.9, 116.1, 58.2 (q, JC—F=27.0 Hz), 35.59 (d, JC—F=1.5 Hz), 21.2, 21.1;19F NMR (376 MHz, CDCl3)δ: -75.4 (d, J=6.6 Hz). HRMS (ESI) calcd for C14H17F3N2ONa[M+Na]+287.1362, found 309.1185.
3.2.2 化合物6的合成
在50 mL的圆底烧瓶中, 依次加入三氟甲基苯乙酮 (5a) (0.034 g, 0.20 mmol)、苯甲酰肼 (2a) (0.033 g, 0.24 mmol)、对甲苯磺酸 (0.007 g, 0.04 mmol) 和THF 3 mL, 将混合物在回流条件下搅拌. TLC检测反应, 5 h后有酰腙大量的生成.此时加入烯丙基溴 (3a) (0.060 g, 0.50 mmol)、铟粉 (0.034 g, 0.30 mmol) 和Ni (ClO)4·6H2O (0.005 g, 0.02 mmol), 继续搅拌回流此反应混合物, TLC检测19 h后反应完全.然后向反应混合物中加入3 mL饱和氯化铵溶液, 搅拌10 min, 用乙酸乙酯萃取 (5 mL×3), 分出有机相, 用无水硫酸镁干燥, 蒸去溶剂后, 进行柱层析[V(石油醚):V(乙酸乙酯)=5:1]分离得6a.化合物6b~6k的合成方法同6a.
N'-(1-三氟甲基-1-苯基丁-3-烯-1-基) 苯甲酰肼 (6a): 55.4 mg, 产率83%.白色固体, m.p. 122~124 ℃; 1H NMR (600 MHz, CDCl3) δ: 7.68 (d, J=7.8 Hz, 2H), 7.65 (d, J=7.2 Hz, 2H), 7.50 (t, J=7.2 Hz, 1H), 7.43 (d, J=7.8 Hz, 2H), 7.40 (d, J=7.8 Hz, 2H), 7.38 (t, J=7.2 Hz, 1H), 7.34 (s, 1H), 5.98 (s, 1H), 5.76~5.69 (m, 1H), 5.18 (d, J=16.8 Hz, 1H), 5.10 (d, J=10.2 Hz, 1H), 3.03 (dd, J=15.0, 6.6 Hz, 1H), 2.95 (dd, J=15.0, 7.2 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 166.5, 134.8, 132.3, 132.0, 131.2, 128.7, 128.6, 128.6, 127.6, 126.8, 126.4 (q, JC—F=286.5 Hz), 119.6, 67.8 (q, JC—F=24.0 Hz), 37.01; 19F NMR (376 MHz, CDCl3)δ: -72.4 (s); HRMS (ESI) calcd for C18-H18F3N2O[M+H]+ 335.1366, found 335.1356.
N'-(1-三氟甲基-1-苯基丁-3-烯-1-基)-4-甲氧基苯甲酰肼 (6b): 64.1 mg, 产率88%.白色固体, m.p. 103~104 ℃; 1H NMR (600 MHz, CDCl3)δ: 7.68 (d, J=7.8 Hz, 2H), 7.62 (d, J=8.4 Hz, 2H), 7.43 (s, 1H), 7.40 (t, J=7.2 Hz, 2H), 7.36 (t, J=7.2 Hz, 1H), 6.87 (d, J=8.4 Hz, 2H), 5.97 (d, J=7.2 Hz, 1H), 5.76~5.69 (m, 1H), 5.16 (d, J=16.8 Hz, 1H), 5.08 (d, J=10.2 Hz, 1H), 3.79 (s, 3H), 3.03 (dd, J=15.0, 6.6 Hz, 1H), 2.95 (dd, J=15.0, 7.2 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 166.4, 162.5, 134.9, 131.3, 128.7, 128.5, 128.4, 127.6, 126.4 (q, JC—F=286.5 Hz), 124.4, 119.3, 113.8, 67.7 (q, JC—F=24.0 Hz), 55.2, 36.7; 19F NMR (376 MHz, CDCl3) δ: -72.5 (s); HRMS (ESI) calcd for C19H20F3N2O2[M+H]+ 365.1471, found 365.1459.
N'-(1-三氟甲基-1-苯基丁-3-烯-1-基)-4-甲基苯甲酰肼 (6c): 59.9 mg, 产率86%.白色固体, m.p. 102~103 ℃; 1H NMR (600 MHz, CDCl3)δ: 7.66 (d, J=7.8 Hz, 2H), 7.53 (d, J=8.4 Hz, 2H), 7.47 (d, J=7.2 Hz, 1H), 7.39 (t, J=7.2 Hz, 2H), 7.34 (t, J=7.2 Hz, 1H), 7.16 (d, J=7.8 Hz, 2H), 5.97 (d, J=7.8 Hz, 1H), 5.74~5.67 (m, 1H), 5.14 (d, J=16.8 Hz, 1H), 5.06 (d, J=10.2 Hz, 1H), 3.02 (dd, J=15.2, 6.6 Hz, 1H), 2.94 (dd, J=15.6, 7.2 Hz, 1H), 2.34 (s, 3H); 13C NMR (150 MHz, CDCl3) δ: 166.6, 142.3, 134.8, 131.2, 129.3, 129.2, 128.5, 128.4, 127.6, 126.8, 126.3 (q, JC—F=286.5 Hz), 119.2, 67.7 (q, JC—F=24.0 Hz), 36.7, 21.2; 19F NMR (376 MHz, CDCl3)δ: -72.5 (s); HRMS (ESI) calcd for C19H20F3N2O[M+H]+ 349.1522, found 349.1511.
N'-(1-三氟甲基-1-苯基丁-3-烯-1-基)-3-甲基苯甲酰肼 (6d): 57.1 mg, 产率82%.白色固体, m.p. 100~101 ℃. 1H NMR (600 MHz, CDCl3) δ: 7.67 (d, J=7.8 Hz, 2H), 7.48 (s, 1H), 7.42~7.35 (m, 5H), 7.30~7.25 (m, 2H), 5.97 (d, J=7.8 Hz, 1H), 5.75~5.68 (m, 1H), 5.16 (dd, J=17.4, 1.8 Hz, 1H), 5.09 (d, J=10.2 Hz, 1H), 3.03 (dd, J=15.6, 7.2 Hz, 1H), 2.94 (dd, J=15.0, 7.2 Hz, 1H), 2.35 (s, 3H); 13C NMR (150 MHz, CDCl3) δ: 166.7, 138.6, 134.8, 132.7, 132.2, 131.2, 128.6, 128.5, 128.5, 127.6, 126.4 (q, JC—F=285.0 Hz), 123.7, 119.5, 116.6, 67.9 (q, JC—F=24.0 Hz), 36.8, 21.2;19F NMR (376 MHz, CDCl3)δ: -72.5 (s); HRMS (ESI) calcd for C19H20F3N2O[M+H]+ 349.1526, found 349.1522.
N'-(1-三氟甲基-1-苯基丁-3-烯-1-基)-4-三氟甲基苯甲酰肼 (6e): 64.3 mg, 产率80%.白色固体, m.p. 94~95 ℃; 1H NMR (600 MHz, CDCl3)δ: 7.71 (d, J=8.4 Hz, 2H), 7.66 (d, J=7.8 Hz, 2H), 7.63 (d, J=7.8 Hz, 2H), 7.58 (d, J=7.2 Hz, 1H), 7.42 (t, J=7.8 Hz, 2H), 7.37 (t, J=7.2 Hz, 1H), 5.96 (d, J=7.8 Hz, 1H), 5.74~5.67 (m, 1H), 5.17 (d, J=17.4 Hz, 1H), 5.10 (d, J=10.2 Hz, 1H), 3.00 (dd, J=15.0, 6.6 Hz, 1H), 2.94 (dd, J=15.0, 7.2 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 165.1, 135.6, 134.6, 133.7 (q, JC—F=33.0 Hz), 130.9, 128.8, 128.7, 127.5, 127.4, 125.7 (q, JC—F=3.0 Hz), 124.9 (q, JC—F=154.5 Hz), 122.6, 119.8, 67.9 (q, J JC—F=24.0 Hz), 37.4; 19F NMR (376 MHz, CDCl3)δ: -63.5 (s), -72.4 (s); HRMS (ESI) calcd for C19H17F6N2O[M+H]+ 403.1240, found 403.1226.
N'-(1-三氟甲基-1-苯基丁-3-烯-1-基)-4-氯苯甲酰肼 (6f): 62.6 mg, 产率85%.白色固体, m.p. 86~88 ℃; 1H NMR (600 MHz, CDCl3)δ: 7.66 (d, J=7.8 Hz, 2H), 7.58 (d, J=7.8 Hz, 1H), 7.55 (d, J=8.4 Hz, 2H), 7.41 (t, J=7.2 Hz, 2H), 7.37 (d, J=7.2 Hz, 1H), 7.33 (d, J=8.4 Hz, 2H), 5.95 (d, J=7.8 Hz, 1H), 5.74~5.67 (m, 1H), 5.16 (d, J=16.8 Hz, 1H), 5.09 (d, J=10.2 Hz, 1H), 3.01 (dd, J=15.0, 6.6 Hz, 1H), 2.94 (dd, J=15.0, 7.2 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 165.6, 138.2, 134.6, 131.1, 130.6, 128.8, 128.6, 128.5, 128.2, 127.5, 126.3 (q, JC—F=286.5 Hz), 119.5, 67.8 (q, JC—F=24.0 Hz), 36.9; 19F NMR (376 MHz, CDCl3)δ: -72.2 (s); HRMS (ESI) calcd for C18H17ClF3N2O[M+H]+369.0976, found 369.0964.
N'-(1-三氟甲基-1-苯基丁-3-烯-1-基)-2-呋喃甲酰肼 (6g): 51.8 mg, 产率80%.白色固体, m.p. 74~75 ℃; 1H NMR (600 MHz, CDCl3)δ: 7.67 (d, J=7.8 Hz, 2H), 7.62 (d, J=6.0 Hz, 1H), 7.42 (d, J=7.2 Hz, 2H), 7.40 (s, 1H), 7.37 (t, J=7.2 Hz, 1H), 7.12 (d, J=3.0 Hz, 1H), 6.49~6.46 (m, 1H), 5.76 (d, J=7.8 Hz, 1H), 5.74~5.68 (m, 1H), 5.18 (d, J=16.8 Hz, 1H), 5.10 (d, J=10.8 Hz, 1H), 3.01 (dd, J=15.6, 7.2 Hz, 1H), 2.93 (dd, J=15.0, 7.2 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 156.9, 146.2, 144.3, 134.7, 131.1, 128.6, 128.5, 127.5, 126.3 (q, JC—F=286.5 Hz), 119.7, 115.1, 112.0, 67.7 (q, JC—F=24.0 Hz), 36.8; 19F NMR (376 MHz, CDCl3) δ: -72.6 (s); HRMS (ESI) calcd for C16H16F3N2O2[M+H]+ 325.1158, found 325.1147.
N'-[1-三氟甲基-1-(3-氯) 苯基丁-3-烯-1-基]苯甲酰肼 (6h): 60.4 mg, 产率82%.白色固体, m.p. 84~85 ℃; 1H NMR (600 MHz, CDCl3)δ: 7.69 (s, 1H), 7.65 (d, J=7.8 Hz, 2H), 7.57 (d, J=4.2 Hz, 1H), 7.50 (t, J=7.2 Hz, 1H), 7.41 (t, J=7.2 Hz, 3H), 7.38~7.31 (m, 2H), 5.93 (d, J=7.2Hz, 1H), 5.74~5.67 (m, 1H), 5.18 (d, J=16.8 Hz, 1H), 5.13 (d, J=10.2 Hz, 1H), 3.01 (dd, J=15.0, 6.6 Hz, 1H), 2.90 (dd, J=15.0, 7.2 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 166.8, 136.9, 134.6, 132.1, 130.7, 129.8, 128.9, 128.7, 128.6, 127.9, 126.9, 126.1 (q, JC—F=286.5 Hz), 125.9, 120.5, 67.6 (q, JC—F=24.0 Hz), 36.9; 19F NMR (376 MHz, CDCl3)δ: -72.3 (s); HRMS (ESI) calcd for C18H17ClF3-N2O[M+H]+ 369.0976, found 369.0965.
N'-[1-三氟甲基-1-(4-甲氧基) 苯基丁-3-烯-1-基]苯甲酰肼 (6i): 64.1 mg, 产率88%.白色固体, m.p. 103~104 ℃; 1H NMR (600 MHz, CDCl3) δ: 7.65 (d, J=7.2 Hz, 2H), 7.58 (d, J=9.0 Hz, 2H), 7.50 (t, J=7.2 Hz, 1H), 7.41 (t, J=7.8 Hz, 2H), 7.33 (d, J=5.4 Hz, 1H), 6.93 (d, J=8.4 Hz, 2H), 5.92 (d, J=7.8 Hz, 1H), 5.77~5.70 (m, 1H), 5.17 (d, J=16.8 Hz, 1H), 5.10 (d, J=10.2 Hz, 1H), 3.82 (s, 3H), 3.01 (dd, J=15.0, 6.6 Hz, 1H), 2.93 (dd, J=15.0, 7.2 Hz, 1H); 13C NMR (150 MHz, CDCl3) δ: 166.4, 159.6, 132.3, 131.9, 131.4, 129.0, 128.7, 126.8, 126.5, 126.4 (q, JC—F=285.6 Hz), 119.5, 113.9, 67.4 (q, JC—F=24.0 Hz), 55.2, 36.6; 19F NMR (376 MHz, CDCl3)δ: -73.1 (s); HRMS (ESI) calcd for C19H19F3N2O2Na[M+Na]+ 387.1291, found 387.1278.
N'-[1-三氟甲基-1-(3-甲基) 苯基丁-3-烯-1-基]苯甲酰肼 (6j): 57.1 mg, 产率82%.白色固体, m.p. 99~101 ℃; 1H NMR (600 MHz, CDCl3) δ: 7.65 (d, J=7.8 Hz, 2H), 7.51~7.48 (m, 2H), 7.46 (d, J=7.8 Hz, 1H), 7.41 (t, J=7.8 Hz, 2H), 7.35 (d, J=7.8 Hz, 1H), 7.30 (t, J=7.8 Hz, 1H), 7.18 (d, J=7.2 Hz, 1H), 5.99 (d, J=7.8Hz, 1H), 5.75~5.68 (m, 1H), 5.17 (d, J=17.4 Hz, 1H), 5.10 (d, J=10.2 Hz, 1H), 3.02 (dd, J=15.0, 6.6 Hz, 1H), 2.94 (dd, J=15.0, 7.2 Hz, 1H), 2.38 (s, 3H); 13C NMR (150 MHz, CDCl3) δ: 166.4, 138.3, 134.7, 132.4, 131.9, 131.3, 129.4, 128.7, 128.5, 128.2, 126.8, 126.4 (q, JC—F=286.5 Hz), 124.6, 119.5, 67.7 (q, JC—F=24.0 Hz), 36.8, 21.6; 19F NMR (376 MHz, CDCl3)δ: -72.8(s); HRMS (ESI) calcd for C19H20F3N2O[M+H]+ 349.1522, found 349.1513.
N'-[1-三氟甲基-1-(3-甲基) 苯基丁-3-烯-1-基]-3-甲基苯甲酰肼 (6k): 57.9 mg, 产率80%.白色固体, m.p. 103~105 ℃; 1H NMR (600 MHz, CDCl3) δ: 7.49 (s, 2H), 7.46 (d, J=7.8 Hz, 1H), 7.41 (d, J=6.6 Hz, 1H), 7.34 (s, 1H), 7.30 (t, J=7.8 Hz, 2H), 7.27 (t, J=7.8 Hz, 1H), 7.18 (d, J=7.8 Hz, 1H), 5.98 (d, J=7.8 Hz, 1H), 5.75~5.68 (m, 1H), 5.16 (d, J=16.8 Hz, 1H), 5.09 (d, J=10.2 Hz, 1H), 3.02 (dd, J=15.0, 6.6 Hz, 1H), 2.94 (dd, J=15.0, 7.2 Hz, 1H), 2.38 (s, 3H), 2.37 (s, 3H); 13C NMR (150 MHz, CDCl3)δ: 166.6, 138.7, 138.3, 134.7, 132.7, 132.3, 131.3, 129.4, 128.6, 128.4, 128.2, 127.6, 126.4 (q, JC—F=286.5 Hz), 124.7, 123.7, 119.4, 67.7 (q, JC—F=24.0 Hz), 36.7, 21.6, 21.3;19F NMR (376 MHz, CDCl3) δ: -72.6 (s); HRMS (ESI) calcd for C20H22F3N2O[M+H]+ 363.1679, found 363.1671.
-
-
[1]
(a) Mishra, M.; Tiwari, K.; Mourya, P. Polyhedron 2015, 89, 29.
(b) Aubin, S.; Martin, B.; Delcros, J. G. J. Med. Chem. 2005, 48, 330.
(c) Huang, R.; Wang, Q. J. Orgnomet. Chem. 2001, 637, 94.
(d) Ke, S.; Sun, T.; Liang, Y. Yang, Z. Chin. J. Org. Chem. 2010, 30, 1820 (in Chinese).
(柯少勇, 孙婷婷, 梁英, 杨自文, 有机化学, 2010, 30, 1820.) http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract339482.shtml -
[2]
(a) Zheng, L. W.; Wu, L. L.; Zhao, B. X.; Dong, W. L.; Miao, J. Y. Bioorg. Med. Chem. 2009, 17 1957.
(b) Lian, S.; Su, H.; Zhao, B. X.; Liu, W. Y.; Zheng, L. W.; Miao, J. Y. Bioorg. Med. Chem. 2009, 17, 7085. -
[3]
Duarte, C. D.; Tributino, J. L. M.; Lacerda, D. I.; Martins, M. V.; Alexandre-Moreira, M. S.; Dutra, F.; Bechara, E. J. H.; De-Paula, F. S.; Goulart, M. O. F.; Ferreira, J.; Calixto, J. B.; Nunes, M. P.; Bertho, A. L.; Miranda, A. L. P.; Barreiroa, E. J.; Fraga, C. A. M. Bioorg. Med. Chem. 2007, 15, 2421. doi: 10.1016/j.bmc.2007.01.013
-
[4]
(a) Formicola, L.; Maréchal, X.; Basse, N.; Bouvier-Durand, M.; Bonnet-Delpon, D.; Milcent, T.; Reboud-Ravaux, M.; Ongeri, S. Bioorg. Med. Chem. 2009, 19, 83.
(b) Onnis, V.; Cocco, M. T.; Fadda, R.; Congiu, C. Bioorg. Med. Chem. 2009, 17, 6158. -
[5]
(a) Wing, K. D. Science 1988, 241, 467.
(b) Wing, K. D.; Slawecki, R. A.; Carlson, G. R. Science 1988, 241, 470. -
[6]
(a) Yoneya, M.; Takada, S.; Maeda, Y.; Yokoyama, H. Liq. Cryst. 2008, 35, 339.
(b) Parra, M.; Hidalgo, P.; Barberá, J.; Carrasco, E.; Saavedra, C. Liq. Cryst. 2006, 33, 391.
(c) Cui, H.; Xu, Y.; Zhang, Z. F. Anal. Chem. 2004, 76, 4002. -
[7]
(a) Licandro, E.; Perdicchia, D. Eur. J. Org. Chem. 2004, 665.
(b) Flagstad, T.; Petersen, M. T.; Nielsen, T. E. Angew. Chem., Int. Ed. 2015, 54, 8395.
(c) Liu, Z.; Zhao, J.; Huang, X. Bioorg. Med. Chem. Lett. 2006, 16, 1828.
(d) Bechara, W. S.; Khazhieva, I. S.; Rodriguez, E.; Charette, A. B. Org. Lett. 2015, 17, 1184.
(e) Gao, Q.; Liu, S.; Wu, X.; Zhang, J.; Wu, A. Org. Lett. 2015, 17, 2960.
(f) Lavergne, K.; Bongers, A.; Betit, L.; Beauchemin, A. M. Org. Lett. 2015, 17, 3612. -
[8]
(a) Alonso, C.; Marigorta, E. M.; Rubiales, G.; Palacios, F. Chem. Rev. 2015, 115, 1847.
(b) Tomashenko, O. A.; Grushin, V. V. Chem. Rev. 2011, 111, 4475.
(c) Ma, J.-A.; Cahard, D. Chem. Rev. 2008, 108, PR1.
(d) Qing, F. Chin. J. Org. Chem. 2012, 32, 815 (in Chinese).
(卿凤翎, 有机化学, 2012, 32, 815.) http://sioc-journal.cn/Jwk_yjhx/CN/abstract/abstract341187.shtml -
[9]
(a) Fustero, S.; Simên-Fuentes, A.; Barrio, P.; Haufe, G. Chem. Rev. 2015, 115, 871.
(b) Nie, J.; Guo, H.-C.; Cahard, D.; Ma, J.-A. Chem. Rev. 2011, 111, 455.
(c) Schlosser, M. Angew. Chem., Int. Ed. 2006, 45, 5432.
(d) Uneyama, K.; Katagiri, T.; Amii, H. Acc. Chem. Res. 2008, 41, 817. -
[10]
(a) Shen, Z.-L.; Wang, S.-Y.; Chok, Y.-K.; Xu, Y.-K.; Loh, T.-P. Chem. Rev. 2013, 113, 271.
(b) Roy, U. K.; Roy, S. Chem. Rev. 2010, 110, 2472.
(c) Zhao, K.; Shen, L.; Shen, Z.-L.; Loh, T.-P. Chem. Soc. Rev. 2017, 46, 586. -
[11]
Issac, M. B.; Chan, T.-H. Tetrahedron Lett. 1995, 36, 8957. doi: 10.1016/0040-4039(95)01982-N
-
[12]
Kornblum, N.; Chen, S. I.; Kelly, W. J. J. Org. Chem. 1988, 53, 1831. doi: 10.1021/jo00243a052
-
[13]
陈文杰, 廖道华, 化学世界, 2006, (5), 285.Chen, W. J.; Liao, D. H. Chem. World 2006, (5), 285 (in Chinese).
-
[1]
-
表 1 合成化合物4a的反应条件优化a
Table 1. Optimization of reaction condition for the synthesis of compound 4a
Entry Molar ratio of 1/2a/3/In Solvent Temp./℃ Isolated yield/% 1 1.5/1/1.5/1.5 THF r.t. 0 2 1.5/1/1.5/1.5 THF 50 45 3 1.5/1/1.5/1.5 THF Reflux 54 4 1.5/1/2/1.5 THF Reflux 75 5 1.5/1/2.5/1.5 THF Reflux 83 6 1.5/1/3/1.5 THF Reflux 85 7 2/1/2.5/1.5 THF Reflux 84 8 1/1/2.5/1.5 THF Reflux 71 9 1/1.5/2.5/1.5 THF Reflux 73 10 1/2/2.5/1.5 THF Reflux 76 11 1.5/1/2.5/1.5 CH2Cl2 Reflux 77 12 1.5/1/2.5/1.5 EtOH Reflux 64 13 1.5/1/2.5/1.5 Toluene Reflux 56 14 1.5/1/2.5/1.5 1, 4-Dioxane Reflux 60 15 1.5/1/2.5/1.5 H2O Reflux 42 a A mixture of 1 (0.15~0.3 mmol), benzoylhydrazine (0.15~0.3mmol), solvent (4.0 mL) was staired at reflux for 3 h, then 3a (0.225~0.0.45 mmol), In powder (0.225 mmol) were added and stair at reflux for another 21 h. 表 2 酰肼的适用范围a
Table 2. Substrate scope of acylhydrazines
Entry R1 Product Isolated yield/% 1 p-MeOC6H4 4b 90 2 p-MeC6H4 4c 83 3 m-MeC6H4 4d 72 4 o-MeC6H4 4e 56 5 p-CF3C6H4 4f 81 6 p-FC6H4 4g 78 7 p-ClC6H4 4h 86 8 m-BrC6H4 4i 85 9 2-Furyl 4j 85 10 Me 4k 0 11 i-C3H7 4l 0 12 n-C17H35 4m 0 a A mixture of 1 (0.3 mmol), acylhydrazine (0.2 mmol) in THF (4.0 mL) was stirred at reflux for 3 h, then 3a (0.5mmol) and In powder (0.3 mmol) were added and stirred at reflux for another 21 h. 表 3 烯丙基溴的适用范围a
Table 3. Substrate scope of allyl bromides
Entry R1 3 Product Isolated yield/% 1 C6H5 3b 4n 80 2 p-MeC6H4 3b 4o 82 3 p-MeOC6H4 3b 4p 86 4 P-FC6H4 3b 4q 74 5 p-ClC6H4 3b 4r 78 6 C6H5 3c 4s 82 b 7 p-MeC6H4 3c 4t 84 b 8 p-MeOC6H4 3c 4u 88 b 9 P-FC6H4 3c 4v 76 b 10 C6H5 3d 4w 83 c 11 p-MeOC6H4 3d 4x 86 d 12 C6H5 3e 0 13 C6H5 3f 4y 81 14 m-MeC6H4 3f 4z 76 a A mixture of 1 (0.3 mmol), acylhydrazine (0.2 mmol) in THF (4.0 mL) was stirred at reflux for 3 h, then 3 (0.5 mmol) and In powder (0.3 mmol) were added and stirred at reflux for another 21 h. b anti/syn=60/40, determined by 19F NMR analyses. c anti/syn=48/52, determined by 19F NMR analyses. d anti/syn=56/44, determined by 19F NMR analyses. 表 4 合成化合物6a的反应条件优化
Table 4. Optimization of the reaction conditions for the synthesis of compound 6a
Entry Molar ratio of 5a/2a/3a/In Catalyst Isolated yield/% 1 1/1.2/2.5/1.5 None 0 2 1/1.2/2.5/1.5 Et2O·BF3 (20%) Trace 3 1/1.2/2.5/1.5 Ni (ClO)4·6H2O (20%) 12 4 1/1.2/2.5/1.5 PTSA (20%) 24 5b 1/1.2/2.5/1.5 PTSA (20%) Ni (ClO)4·6H2O (10%) 83 a A mixture of 5a (0.2 mmol), 2a (0.24 mmol), catalyst (20%, 0.04 mmol) in THF (3.0 mL) was stirred at reflux for 5 h, then 3a (0.5 mmol), In powder (0.3 mmol) were added and stirred at reflux for another 19 h. b 5a (0.2 mmol), 2a (0.24 mmol), PTSA (20%, 0.04 mmol), in THF (3.0 mL), after stirred at reflux for (5 h), then Ni (ClO)4·6H2O (10%, 0.02 mmol), 3a (0.5 mmol), In powder (0.3 mmol) were stirred at reflux for another 19 h. 表 5 三氟甲基酮和酰肼的适用范围a
Table 5. Substrate scope of trifluoromethylketones and acylhydrazines
Entry R1 R4 Product Isolated yield/% 1 p-MeOC6H4 Ph 6b 88 2 p-MeC6H4 Ph 6c 86 3 m-MeC6H4 Ph 6d 82 4 p-CF3C6H4 Ph 6e 80 5 p-ClC6H4 Ph 6f 85 6 2-Furyl Ph 6g 80 7 Ph m-ClC6H4 6h 82 8 Ph p-MeOC6H4 6i 88 9 Ph m-MeC6H4 6j 82 10 m-MeC6H4 m-MeC6H4 6k 83 12 Me Ph 6l 0 13 n-C17H35 Ph 6m 0 a A mixture of 5a (0.2 mmol), acylhydrazines (0.24 mmol), PTSA (20%, 0.04 mmol), in THF (3.0 mL) was stirred at reflux for 5 h, then Ni (ClO)4·6H2O (10%, 0.02 mmol), 3a (0.5 mmol) and In powder (0.3 mmol) were added and stirred at reflux for another 19 h. -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 9
- 文章访问数: 1148
- HTML全文浏览量: 106


 下载:
下载:
 下载:
下载:

