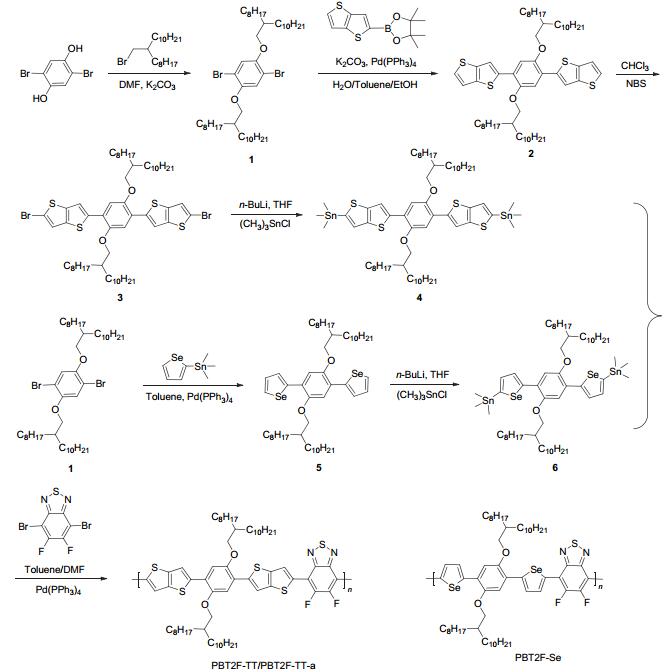
 Figure Scheme 1.
Synthetic routes of PBT2F-TT-a, PBT2F-TT and PBT2F-Se
Figure Scheme 1.
Synthetic routes of PBT2F-TT-a, PBT2F-TT and PBT2F-Se

综合研究DPE添加剂对含5, 6-二氟-苯并[1, 2, 5]噻二唑给-受体共聚物的光伏性能影响
English
Comprehensive Study of the Effect of DPE Additive on Photovoltaic Performance of 5, 6-Difluoro-benzo[1, 2, 5]thiadiazole Based Donor-acceptor Copolymers
-
1 Introduction
Since Tang[1] reported the two-layer organic photovoltaic cell and demonstrated its great potential for harnessing low-cost solar energy in 1986, much progress has been made in the optimization of device architectures. Recently bulk heterojunction (BHJ) type organic photovoltaics (OPVs) developed by Heeger et al.[2] are getting more popular for two reasons: enlarged donor/acceptor interface area and nanoscale pathways for charge transport. During the last decades the polymer/fullerene derivative, [3] small molecule/fullerene derivative, [4] polymer/non-fullerene small molecule, [5] and polymer/polymer[6] blends were the mainstream photoactive layers in BHJ organic solar cells. Remarkably, by the judicious design of donor and acceptor materials and the optimization of device fabrication conditions, the power conversion efficiency (PCE) of single-junction BHJ device has now surpassed 11%, [7] which brings OPVs one big step closer to commercialization.
So far tremendous research efforts have been devoted to the design and synthesis of novel donor (D) and acceptor (A) materials aiming at higher PCEs. A broad absorption spectrum with high molar extinction coefficient for the donor/acceptor blend is a prerequisite for an efficient BHJ OPV. D-A type donor polymers generally exhibited an intense absorption in the visible range due to the intra-molecular charge transfer (ICT) interaction within their conjugated backbone.[8] The donor units used in the D-A donor polymers are mainly thiophene/selenophene derivatives, and the acceptor units such as 5, 6-difluoro-benzo-[1, 2, 5] thiadiazole (BT2F), [9] diketopyrrolopyrrole (DPP), [10] fluorinated benzotriazole (FTAZ)[7f, 11] and fluorinated thieno[3, 4-b]thiophene[12] have been successfully applied in state-of-the-art donor materials. For example Yan and coworkers[7b] achieved high PCE up to 11.7% with a BT2F-based polymer (PffBT4T-C9C13) in a hydrocarbon processing system. Importantly, You et al.[9c] revealed the importance of introduce of fluorine atoms into electron-deficient motif for the OPVs, where the suppression of recombination losses accounted for the impressive PCEs.
Since the morphology and charge mobility of the blend film are highly susceptible to the intermolecular orientation of conjugated polymers and the interaction with the acceptor materials, [13] the chain planarity that leads to the strong inter-chain π-π stacking is one of the important issues for molecular design of conjugated donor materials. Thus the use of intra-chain non-covalent coulombic interactions such as F…S, S…O, N…H, and F…H could be one of the effective strategies to minimize the torsional angles.[14] Woo et al.[15] reported a series of donor polymers with strong intra-chain interactions exhibiting ordered film morphology and high hole and electron mobilities. Herein we reported the synthesis of three D-A donor polymers (denoted by PBT2F-TT-a, PBT2F-TT, and PBT2F-Se) based on BT2F unit. PBT2F-TT-a and PBT2F-TT were synthesized with the same donor and acceptor monomers, but PBT2F-TT-a has a relatively low molecular weight. In PBT2F-TT-a and PBT2F-TT, phenyl rings flanked with thieno[3, 2-b]thiophene were used as donor monomers, and the central benzene ring was further decorated by two alkoxyl groups to achieve the chain planarity. In PBT2F-Se, selenophene was applied as donor unit, and the intermolecular Se…Se interaction would favor the inter-chain charge transport.[16] In this paper, the photovoltaic performance of the polymer was enhanced greatly by the addition of DPE, which was interrogated by AFM, TEM, alternating current impedance spectrometry, and space-charge-limited current analysis. The result indicated that the improved charge carrier transport and balanced hole/electron mobilities accounted for, in part, the enhancement in Jsc and PCE.
2 Results and discussion
2.1 Synthesis
The synthesis of PBT2F-TT-a, PBT2F-TT, and PBT2F-Se was outlined in Scheme 1. The polymers were synthesized via Stille coupling of 4, 7-dibromo-5, 6-difluoro-benzo[1, 2, 5]thiadiazole with the corresponding donor monomers, and purified by soxhlet extraction. The Mn and PDI were determined by high temperature GPC at 150 ℃ in 1, 2, 4-trichlorobenzene against polystyrene standards. PBT2F-TT showed a relatively higher Mn (10.36 kDa) than that of PBT2F-TT-a (6.79 kDa), which is accomplished by increasing the reaction time for the synthesis of PBT2F-TT. The thermal stability of these three polymers was studied by thermal gravimetric analysis under N2. PBT2F-TT-a and PBT2F-TT showed good thermal stability with the decomposition temperature of 5% weight loss over 300 ℃, and their selenophene analogue PBT2F-Se was decomposed at a relatively low temperature (292 ℃).
The 1H, 13C NMR and MALDI-TOF MS spectra of precursors and monomers (Figures S1~S15), and thermal gravimetric analysis (TGA) (Figure S16) curves are given in the supporting information.
2.2 Optical and electrochemical properties
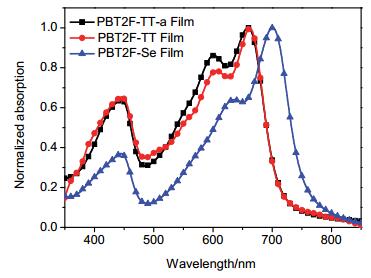
The three polymers revealed similar and strong absorption in the range of 500~700 nm in o-dichlorobenzene solution as shown in Figure S17. Since PBT2F-TT possesses a longer effective conjugated backbone than PBT2F-TT-a, it is not surprising that there is a red-shift of ca. 8 nm in absorption spectrum of PBT2F-TT relative to that of PBT2F-TT-a. However the difference in their solu-tion absorption properties was compensated by the strong inter-molecular interaction in the film state (Figure 1), and the absorption onsets were of the same value (716 nm) for both polymers. PBT2F-Se showed a clear red-shift in maximum peak value (38 nm) as well as the absorption onset (22 nm) as compared to PBT2F-TT, which would be attributed, in part, to the lower-energy LUMO of selenophene motif.[17]
Cyclic voltammetry measurement was carried out to determine the energy levels of these polymers (Figure S18). The oxidation potentials were calibrated with the ferrocene/ferrocenium (Fc/Fc+) redox couple for the estimation of the EHOMO of polymers. The three polymers exhibited similar EHOMO within the range from -5.35 to -5.40 eV. Using -4.3 eV for the ELUMO of PCBM, [18] a relatively high VOC (above 0.7 V) is anticipated. The ELUMO was calculated from the difference between EHOMO and the optical band gap (Table 1). The relatively large difference in the ELUMOof the polymers (-3.62 to -3.69 eV) and PCBM would provide an internal electric field that is strong enough to separate the excitons.[5c]
Polymer ΔT5%/℃ λsolutiona/nm λfilm/nm λonsetb/nm Egc/eV HOMOd/eV LUMOe/eV PBT2F-TT-a 339 650, 598, 439 658, 600, 446 716 1.73 -5.40 -3.67 PBT2F-TT 340 661, 600, 443 662, 603, 447 716 1.73 -5.35 -3.62 PBT2F-Se 292 686, 630, 438 700, 631, 443 738 1.68 -5.37 -3.69 a Measured in dilute o-dichlorobenzene at room temperature. b Measured in the thin film. c Calculated from the absorption edge of the thin film. d Determined from cyclic voltammetry measurement (on conductive ITO substrate). e LUMO=HOMO+Eg. Table 1. Properties of PBT2F-TT-a, PBT2F-TT and PBT2F-Se2.3 Photovoltaic performances
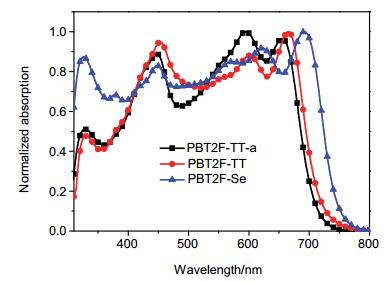
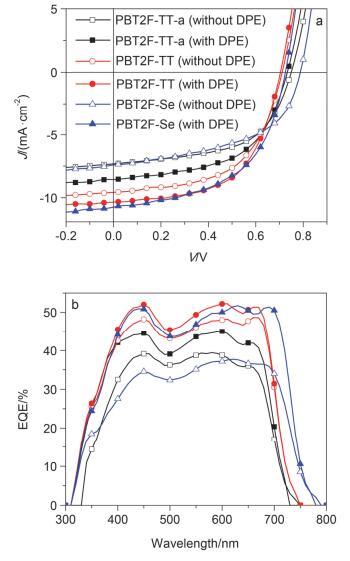
In order to explore the photovoltaic properties of PBT2F-TT-a, PBT2F-TT and PBT2F-Se, PSCs with a device architecture of ITO/PEDOT:PSS/polymer: PC71BM/Ca/Al were fabricated. The polymer:PC71BM blends with different weight ratio (1.5:1, 1:1 and 1:1.5) were dissolved in o-DCB and spin-coated on the ITO/PEDOT:PSS anode. The J-V curves and EQE profiles of these devices are shown in Figures S19~S21. It turns out that the optimum weight ratios are 1.5:1, 1:1 and 1:1 for PBT2F-TT-a:PC71BM, PBT2F-TT:PC71BM and PBT2F-Se:PC71BM blends, respectively. As shown in Figure 2, the polymer:PC71BM blends showed pronounced absorption all through the visible range due to the complementary effect in the light-harvest of donor and acceptor materials. In order to optimize the morphology of the active layer, diphenyl ether (DPE), [10a] a high-boiling-point compound, was applied to improve the film morphology. Initially 1, 8-diiodooctane (DIO) was applied as an additive, [3b] however, the addition of DIO didn't lead to the enhancement in PCE (Figure S22). The device parameters of VOC, JSC, FF, and PCE with and without DPE additive are summarized in Table 2. The data showed that the addition of DPE into the three polymer:PC71BM blends led to remarkable enhancement of their PCEs. For example, PBT2F-Se:PC71BM blend demonstrated significant increase in JSC and FF and slight decrease in VOC after the addition of 2% DPE, giving rise to the increase in PCE from 3.01% to 4.18%. To reveal the influence of molecular weight, the devices performance based on PBT2F-TT-a and PBT2F-TT were investigated. A notable enhancement in the photovoltaic performance was observed with the high-molecular-weight PBT2F-TT. The PCE was increased from 3.48% to 4.22% as the molecular weight was increased from 6.79 kDa to 10.36 kDa. This observation of molecular weight effect on photovoltaic performance was in good agreement with the previous literature.[19] As shown in Figure 3, the EQE curves of these devices indicated a broad photo-response in the visible range. Particularly, PBT2F-Se:PC71BM blend showed a photo-response up to 780 nm, which is coincident with its absorption spectrum.
 Table 2.
Optimized photovoltaic properties of the PSCs based on different polymers/PC71BM blends under the illumination of AM1.5G, 100 mW/cm2
Table 2.
Optimized photovoltaic properties of the PSCs based on different polymers/PC71BM blends under the illumination of AM1.5G, 100 mW/cm2
Polymera DPE additive VOC/V JSC/(mA•cm-2) FF/% PCEmax/% PCEave/% PBT2F-TT-a - 0.74 7.32 56.1 3.10 3.06 PBT2F-TT-a 2% 0.73 8.56 55.5 3.48 3.41 PBT2F-TT - 0.71 9.50 56.3 3.82 3.80 PBT2F-TT 2% 0.70 10.44 57.2 4.22 4.15 PBT2F-Se - 0.78 7.42 51.5 3.01 2.94 PBT2F-Se 2% 0.72 10.75 53.7 4.18 4.15 aThe polymer:PC71BM weight ratios were 1.5:1 (w/w), 1:1 (w/w) and 1:1 (w/w) for PBT2F-TT-a, PBT2F-TT and PBT2F-Se, respectively. Table 2. Optimized photovoltaic properties of the PSCs based on different polymers/PC71BM blends under the illumination of AM1.5G, 100 mW/cm22.4 Morphology study
In order to study the effect of DPE on surface morphology of the active layer, AFM adhesion imaging was carried out.[20] As revealed in Figure 4, the polymer:PC71BM blend films without DPE developed continuous networks, which would lead to the efficient charge transport. The morphology of neat films of the three polymers has also been investigated, and all neat films of these polymers showed fibrillar structures (Figure S23). No significant change was detected for PBT2F-TT-a:PC71BM and PBT2F-Se: PC71BM blends after the addition of DPE. It is worthy to note that PBT2F-TT:PC71BM blend film with DPE, as compared to the one without DPE, is featured with small scale segregation, which could provide substantial interfacial area for charge separation and lead to the increase in JSC. TEM measurement was also performed to probe the phase separation of these blend films with and without DPE. As showed in Figure S24, the addition of DPE didn't change the morphologies of PBT2F-TT-a:PC71BM and PBT2F-TT:PC71BM blends greatly. PBT2F-Se:PC71BM blend without DPE showed a weak phase separation (Figure S24c). However PBT2F-Se:PC71BM blend with DPE exhibited a nanoscale phase separation dominated with fibrillar structures, which resulted in the large increase in PCE.
2.5 ACIS, SCLC and JSC-Plight measurement
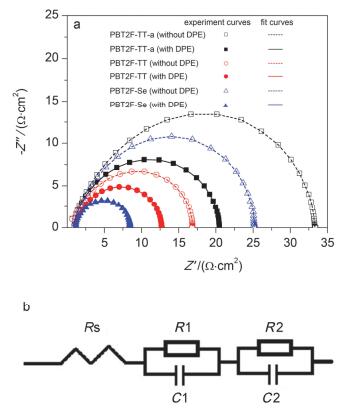
To obtain further insight into the morphological changes and electrical properties of the active layers, the ACIS measurement was conducted in the dark (Figure 5a). The equivalent circuits (Figure 5b) were applied to fit the Nyquist plot.[21] Rs corresponds to the contribution of the resistance from the electrodes. R1 and C1 are related to the active layers, and R2 and C2 correspond to the interfaces between the electrodes and the active layers. Table 3 shows the simulation results of each element. For the PBT2F-TT-a:PC71BM blend, the value of R1 changes slightly after the addition of DPE, which agrees well with the observation of small variation in the surface morphology and phase separation of the blends with and without DPE. While for the PBT2F-Se:PC71BM blend, the value of R1 decreasing remarkably from 3.16 to 1.78 Ω•cm2 may arise from the development of nanoscale phase separation (Figure S24c'). SCLC measurement was also performed to reveal the importance of DPE in governing the bulk hole and electron mobilities of polymer:PC71BM blends (Figure S25). Their charge carrier properties were illustrated in Table 4. All the blends showed enhanced hole and electron mobilities after the addition of DPE, which accounted for the increase in JSC and PCE. Notably, the PBT2F-TT: PC71BM blend under optimal conditions demonstrated well-balanced charge transport with hole mobility of 1.34×10-5 cm2/(V•s) and electron mobility of 9.72×10-6cm2/(V•s), which also contributes to the enhancement in JSC and FF.[22] The dependence of JSC on various light intensities was investigated to study the charge recombination dynamics of the devices. The correlation between JSC and light intensity (Plight) can be expressed as a power-law equation of JSC∝Plightα.[23] The α value for the device without and with DPE were approaching to 1, which might suggest the weak bimolecular recombination in these blend films (Figure S26).
 Table 3.
The parameters of the equivalent circuits for the PSCs operated in the dark near the corresponding VOC bias voltages
Table 3.
The parameters of the equivalent circuits for the PSCs operated in the dark near the corresponding VOC bias voltages
Polymer DPE additive Rs/(Ω•cm2) R1/(Ω•cm2) C1/(F•cm-2) R2/(Ω•cm2) C2/(F•cm-2) PBT2F-TT-a — 1.08 5.86 1.63×10-10 26.01 2.23×10-10 PBT2F-TT-a 2% 1.05 5.76 2.21×10-10 13.54 3.82×10-10 PBT2F-TT — 1.01 5.63 1.48×10-10 10.13 2.99×10-10 PBT2F-TT 2% 1.15 2.80 2.77×10-10 8.67 3.85×10-10 PBT2F-Se — 1.07 3.16 2.70×10-10 20.72 2.29×10-10 PBT2F-Se 2% 1.12 1.78 3.29×10-10 5.54 3.59×10-10 Table 3. The parameters of the equivalent circuits for the PSCs operated in the dark near the corresponding VOC bias voltagesPolymer DPE additive Hole μ/(cm2•V-1•s-1) Electron μ/(cm2•V-1•s-1) PBT2F-TT-a — 1.53×10-5 2.12×10-4 PBT2F-TT-a 2% 2.13×10-5 2.72×10-4 PBT2F-TT — 1.30×10-5 6.46×10-6 PBT2F-TT 2% 1.34×10-5 9.72×10-6 PBT2F-Se — 4.86×10-6 8.12×10-5 PBT2F-Se 2% 8.86×10-6 8.22×10-5 Table 4. SCLC mobilities of the devices3 Conclusions
In conclusion, three D-A polymers (PBT2F-TT-a, PBT2F-TT, and PBT2F-Se) were synthesized, characterized and applied as donor materials for organic photovoltaics. These polymers showed complementary absorption with PC71BM in the visible range, which could contribute to their relatively high PCEs. The surface morphologies of polymer:PC71BM blends as revealed by AFM measurement are featured with continuous network, which would facilitate charge transport. The correlation of DPE additive and the increase in PCE of these polymer-based devices were revealed by ACIS, SCLC and JSC-Plight measurement. PBT2F-Se:PC71BM blend demonstrated nano-scale phase separation after the addition of DPE. As a result, the resistance of this active layer was reduced greatly, and the hole and electron mobilities were enhanced accordingly, which led to a good device performance with JSC of 10.75 mA/cm2, VOC of 0.72 V and PCE of 4.18%.
4 Experimental section
4.1 Measurements and characterization
1H NMR spectra were recorded on a Bruker 400 MHz instrument and 13C NMR spectra were recorded on Agilent DD2-600 MHz NMR instrument, and the spectra were obtained in CDCl3. The matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectra were recorded on a Bruker ultraflextreme MALDI-TOF spectrometer. UV-vis spectra were recorded on a Cary 60 spectrometer (Agilent Technologies) with 1 cm cuvettes. Cyclic voltammograms (CV) were recorded on a CHI600 voltammetric analyzer at room temperature under N2 atmosphere. Electrochemical grade tetrabutylammonium hexafluorophosphate was employed as the supporting electrolyte without further purification. A thin film of the polymer was coated on the conductive ITO plate, which was applied as the working electrode. A platinum wire was used as auxiliary electrode and a Ag wire as reference electrode. High temperature gel permeation chromatography (GPC) was conducted on Agilent PL-GPC220.
4.2 Photovoltaic device fabrication and characterization
The photovoltaic devices with a configuration of ITO/PEDOT:PSS/polymer:PC71BM/Ca/Al were fabricated. ITO substrates were cleaned in detergent, deionized water, acetone, ethanol and isopropanol each for 20 min under ultrasonic washing unit, and treated with ultraviolet-ozone for 15 min after the substrates were completely dried in nitrogen atmosphere. PEDOT:PSS (Clevios P VP AI 4083) was spin-coated on the substrates at 5000 r/min after the solution being passed through 0.45 μm syringe filter, and then annealed at 150 ℃ for 15 min. Later on, the substrates were transferred into a glove box filled with N2. The active layer of PBT2F-TT:PC71BM, PBT2F-TT-a: PC71BM and PBT2F-Se:PC71BM were dissolved in o-DCB with 2% DPE as additive, and then were spin-coated on the PEDOT:PSS surface to form about 80, 90 and 60 nm films, respectively. At last, 20 nm of Ca and 80 nm of Al were deposited on the active layers under the high vacuum of ca. 10-4Pa. The current density-voltage (J-V) characteristics were measured using a Keithley 2400 Source Measure Unit, in a glove-box under air mass 1.5 global (AM 1.5G) illumination from a solar simulator (SAN-Electric Co., Ltd.) with irradiation intensity of 100 mW/cm2. External quantum efficiency (EQE) was measured using QE-R3011 from Enli Technology Co., Ltd by illuminating the sample with a monochromatic light. The alternating-current impedance spectroscopy (ACIS) measurements were performed with an AC signal with root mean square amplitude of 10 mV over the frequency range of 1 Hz~1 MHz in the dark on IM6 electrochemical workstation (ZAHNER ZENNIUM, Germany). The space-charge-limited current (SCLC) measurement were conducted on hole-and electron-only devices with structure of ITO/PEDOT:PSS/Polymer:PC71BM/MoO3/Al and ITO/PEI/Polymer:PC71BM/Ca/Al, respectively. The charge mobility of a single carrier SCLC device was estimated by Mott-Gurney law equation: J=9ε0εrμV2/8d3, where ε0εr is the permittivity of the active layer, μ the carrier mobility, V the applied voltage, and d the thickness of the active layer. Atomic force microscopy (AFM) images were obtained on a Multimode 8 microscope (Bruker Co.) with peak force quantitative nanomechanical mode to study the surface morphology of Polymer/PC71BM blend films.
4.3 Materials and synthetic procedures
All chemicals were purchased from either J & K Scientific, Alfa Aesar, Acros, or Strem chemicals, and were used without further purification. 1, 4-Dibromo-2, 5-bis[(2-oc-tyldodecyl)oxy] benzene (1), [24] 4, 4, 5, 5-tetramethyl-2-(thieno[3, 2-b]thiophen-2-yl)-1, 3, 2-dioxaborolane, [25] andtrimethyl(selenophen-2-yl)stannane[26]were synthesized according to literature. All air-and moisture-sensitive reactions were performed under a nitrogen atmosphere. Dry tetrahydrofuran (THF), toluene and N, N-dimethylforma-mide (DMF) were obtained from an Innovative Technology solvent purification system.
Synthesis of 2, 5-bis-(2-octyl-dodecyloxy)-1, 4-di(thieno-[3, 2-b]thiophen-2-yl)-benzene (2)
4, 4, 5, 5-Tetramethyl-2-(thieno[3, 2-b]thiophen-2-yl)-1, 3, 2-dioxaborolane (1.5 g, 5.63 mmol), 1, 4-dibromo-2, 5-bis-(2-octyl-dodecyloxy)-benzene (2 g, 2.45 mmol), K2CO3 (1.38 g, 10 mmol), Pd(PPh3)4 (0.11 g, 0.1 mmol) were added into a mixture of toluene (50 mL), deionized water (10 mL) and ethanol (5 mL) under N2. The mixture was heated at 120 ℃ for 72 h. After the removal of solvent, the residue was purified by column chromatography (SiO2, PE) to afford white solid, i.e. compound 2 (1.5 g, 65%). 1H NMR (CDCl3, 400 MHz) δ: 7.74 (s, 2H), 7.36 (d, J=5.2 Hz, 2H), 7.25 (s, 4H), 3.99 (d, J=5.4 Hz, 4H), 1.94~1.86 (m, 2H), 1.24 (s, 64H), 0.87 (t, J=6.3 Hz, 12H); 13C NMR (CDCl3, 151 MHz) δ: 149.82, 141.79, 139.70, 139.55, 126.91, 123.68, 119.59, 118.09, 113.06, 72.68, 38.32, 32.07, 31.70, 30.20, 29.81, 29.51, 27.09, 22.84, 14.28. MALDI-TOF m/z: calcd for C58H90O2S4 [M+] 946.582, found 946.220.
Synthesis of 1, 4-bis(5-bromothieno[3, 2-b]thiophene-2-yl)-2, 5-bis(2-octyl-dodecyloxy)-benzene (3)
To a solution of 2 (1.5 g, 1.6 mmol) in CHCl3 (50 mL), NBS (0.57 g, 3.2 mmol) was added in small portions within 30 min. The mixture was stirred at room temperature overnight. After the removal of solvent, the crude product was purified by column chromatography (SiO2, PE) to afford white solid, i.e. compound 3 (1.7 g, 96%). 1H NMR (CDCl3, 400 MHz) δ: 7.63 (s, 2H), 7.2~7.26 (m, 2H), 7.22 (s, 2H), 3.98 (s, 4H), 1.93~1.86 (m, 2H), 1.25 (s, 64H), 0.87 (d, J=6.5 Hz, 12H); 13C NMR (CDCl3, 151 MHz) δ: 149.72, 141.01, 139.63, 138.61, 123.33, 122.34, 117.47, 113.15, 112.73, 72.71, 38.30, 32.08, 31.70, 30.20, 29.82, 29.53, 27.09, 22.86, 14.29. MALDI-TOF m/z: calcd for C58H88Br2O2S4 [M+] 1104.401, found 1104.108.
Synthesis of 2, 5-bis(2-octyl-dodecyloxy)-1, 4-bis(5-trime-thylstannylthieno[3, 2-b]thiophene-2-yl)-benzene (4)
To a solution of 3 (0.5 g, 0.45 mmol) in anhydrous THF (50 mL), n-butyllithium (0.6 mL, 1.6 mol/L, 0.96 mmol) was added dropwise at -78 ℃. After the mixture was stirred for 2 h at -78 ℃, chlorotrimethylsilane (1.1 mL, 1 mol/L, 1.1 mmol) was added dropwise. Then the cooling bath was removed and the reactant was allowed to warm to room temperature, followed by overnight stirring. The reaction was quenched with addition of ethyl acetate. The product was extracted by chloroform and washed with saturated sodium bicarbonate. After the removal of solvent, the crude product was purified by re-crystallization in ethanol to afford white solid, i.e. compound 4 (0.5 g, 88%). 1H NMR (CDCl3, 400 MHz) δ: 7.74 (s, 2H), 7.28 (s, 2H), 7.24 (s, 2H), 3.99 (d, J=5.2 Hz, 4H), 1.95~1.87 (m, 2H), 1.25 (d, J=7.1 Hz, 64H), 0.87 (t, J=6.4 Hz, 12H), 0.42 (s, 18H); 13C NMR (CDCl3, 151 MHz) δ: 149.85, 145.29, 141.76, 141.62, 141.02, 126.66, 123.80, 117.72, 112.95, 72.59, 38.33, 32.08, 31.71, 30.20, 30.02~29.64, 29.53, 27.11, 22.85, 14.29, -8.03. MALDI-TOF m/z: calcd for C64H106O2S4Sn2 [M+] 1274.512, found 1274.222.
Synthesis of 2, 5-bis-(2-octyl-dodecyloxy)-1, 4-di(sele-nophen-2-yl)-benzene (5)
Trimethyl(selenophen-2-yl)stannane (1.5 g, 5.1 mmol), 1 (1.88 g, 2.3 mmol), and Pd(PPh3)4(0.11 g, 0.1 mmol) were added into a mixture of toluene (100 mL) and DMF (20 mL) under N2. This mixture was heated at 120 ℃ for 48 h. After the removal of solvent, the residue was purified by column chromatography (SiO2, PE) to afford yellowish oil, i.e. compound 5 (1.5 g, 73%). 1H NMR (CDCl3, 400 MHz) δ: 8.06 (d, J=5.6 Hz, 2H), 7.70 (d, J=3.5 Hz, 2H), 7.37~7.32 (m, 2H), 7.31 (s, 2H), 4.00 (d, J=5.4 Hz, 4H), 1.94 (d, J=5.9 Hz, 2H), 1.40~1.21 (m, 64H), 0.87 (t, J=6.4 Hz, 12H); 13C NMR (CDCl3, 151 MHz) δ: 148.91, 143.43, 131.77, 128.98, 126.17, 124.69, 111.41, 72.67, 38.37, 32.08, 31.65, 30.19, 29.80, 29.51, 27.06, 22.85, 14.28. MALDI-TOF m/z: calcd for C54H90O2Se2 [M+] 929.232, found 929.134.
Synthesis of 2, 5-bis(2-octyl-dodecyloxy)-1, 4-bis(5-trime-thylstannylselenophen-2-yl)-benzene (6)
To a solution of 5 (0.85 g, 0.91 mmol) in anhydrous THF (50 mL), n-butyllithium (1.7 mL, 1.6 mol/L, 2.72 mmol) was added dropwise at -78 ℃. After the mixture was stirred for 2 h at -78 ℃, chlorotrimethylsilane (3.2 mL, 1 mol/L, 3.2 mmol)) was added dropwise. Then the cooling bath was removed and the reactant was allowed to warm to room temperature, followed by overnight stirring. The reaction was quenched with the addition of ethyl acetate. The product was extracted by DCM and washed with water. The collected organic layers were combined and dried over magnesium sulfate to afford yellowish oil, i.e. compound 6 (0.8 g, 73%). 1H NMR (CDCl3, 400 MHz) δ: 7.79 (d, J=3.5 Hz, 2H), 7.50 (d, J=3.5 Hz, 2H), 7.26 (s, 2H), 3.96 (d, J=5.6 Hz, 4H), 1.94 (t, J=5.9 Hz, 2H), 1.40~1.13 (m, 64H), 0.87 (t, J=6.5 Hz, 12H), 0.46~0.27 (m, 18H); 13C NMR (CDCl3, 151 MHz) δ: 149.32, 148.91, 146.04, 137.42, 127.73, 124.85, 111.75, 72.70, 38.43, 32.09, 31.72, 30.28, 29.85, 29.53, 27.19, 22.85, -7.83. MALDI-TOF m/z: calcd for C60H106O2Se2Sn2 [M+] 1254.846, found 1254.238.
Synthesis of PBT2F-TT-a and PBT2F-TT
4, 7-Dibromo-5, 6-difluoro-benzo[1, 2, 5]thiadiazole (0.09 g, 0.27 mmol), 4 (0.35 g, 0.27 mmol) and Pd(PPh3)4 (0.015 g, 0.014 mmol) were added into a mixture of toluene (10 mL) and DMF (1 mL) under N2. This mixture was heated at 110 ℃ for 48 h. After cooling down to room temperature, the polymer was precipitated by addition of an excess amount of methanol and then collected by filtration. The crude product was successively extracted with methanol, hexane, DCM and chloroform. The chloroform fraction was concentrated and precipitated by addition of methanol. PBT2F-TT-a was collected by filtration and dried under vacuum (230 mg, 66%). Elemental anal. calcd for C66H94F2N2O2S5: C 68.77, H 8.12, N 2.51; found C 66.07, H 7.56, N 2.40. The number average molecular weight (Mn) and the polydispersity index (PDI) of PBT2F-TT-a estimated by GPC at 150 ℃ in 1, 2, 4-trichlorobenzene are 6.79 kDa and 2.68, respectively. PBT2F-TT was synthesized by the procedure similar to that used for synthesizing PBT2F-TT-a except that the reaction time was increased from 48 h to 72 h (140 mg, 67%). Elemental anal. calcd for C66H94F2N2O2S5: C 68.77, H 8.12, N 2.51; found C 67.27, H 7.62, N 2.38. The Mn and the PDI of PBT2F-TT estimated by GPC at 150 ℃ in 1, 2, 4-trichlorobenzene are 10.36 kDa and 6.27, respectively.
Synthesis of PBT2F-Se
4, 7-Dibromo-5, 6-difluoro-benzo[1, 2, 5]thiadiazole (0.09 g, 0.28 mmol), 6 (0.35 g, 0.28 mmol) and Pd(PPh3)4 (0.015 g, 0.014 mmol) were added into a mixture of toluene (20 mL) and DMF (2 mL) under N2. The mixture was heated at 110 ℃ for 72 h. After cooling down to room temperature, the polymer was precipitated by addition of an excess amount of methanol and then collected by filtration. The crude product was successively extracted with methanol, hexane and DCM. The DCM fraction was concentrated and precipitated by addition of methanol. PBT2F-Se was collected by filtration and dried under vacuum (230 mg, 72%). Elemental anal. calcd for C60H90F2N2O2SSe2: C 65.55, H 8.25, N 2.55; found C 65.29, H 7.91, N 2.48. The Mn and the PDI of PBT2F-Se estimated by GPC at 150 ℃ in 1, 2, 4-trichlorobenzene are 38.56 kDa and 2.19, respectively.
-
-
[1]
Tang, C. W. Appl. Phys. Lett. 1986, 48, 183. doi: 10.1063/1.96937
-
[2]
Yu, G.; Gao, J.; Hummelen, J. C.; Wudl, F.; Heeger, A. J. Science 1995, 270, 1789. doi: 10.1126/science.270.5243.1789
-
[3]
(a) He, Z. ; Zhong, C. ; Su, S. ; Xu, M. ; Wu, H. ; Cao, Y. Nat. Photon. 2012, 6, 591; (b) Huang, Y. ; Kramer, E. J. ; Heeger, A. J. ; Bazan, G. C. Chem. Rev. 2014, 114, 7006; (c) Zhang, S. ; Ye, L. ; Zhao, W. ; Yang, B. ; Wang, Q. ; Hou, J. Sci. China Chem. 2015, 58, 248; (d) Peng, Q. ; Liu, X. ; Su, D. ; Fu, G. ; Xu, J. ; Dai, L. Adv. Mater. 2011, 23, 4554; (e) Yao, H. ; Ye, L. ; Zhang, H. ; Li, S. ; Zhang, S. ; Hou, J. Chem. Rev. 2016, 116, 7397; (f) Cheng, Y. J. ; Yang, S. H. ; Hsu, C. S. Chem. Rev. 2009, 109, 5868; (g) Qin, R. ; Geng, F. ; Wang, D. ; Yao, X. Chin. J. Org. Chem. 2015, 35, 2583 (in Chinese). (秦瑞平, 耿凡, 王丹丰, 姚小静, 有机化学, 2015, 35, 2583. ); (h) Liu, T. ; Pan, X. ; Meng, X. ; Liu, Y. ; Wei, D. ; Ma, W. ; Huo, L. ; Sun, X. ; Lee, T. H. ; Huang, M. ; Choi, H. ; Kim, J. Y. ; Choy, W. C. H. ; Sun, Y. Adv. Mater. 2017, 29, 1604251.
-
[4]
(a) Sun, Y.; Welch, G. C.; Leong, W. L.; Takacs, C. J.; Bazan, G. C.; Heeger, A. J. Nat. Mater. 2012, 11, 44; (b) Xu, S.; Zhou, Z.; Fan, H.; Ren, L.; Liu, F.; Zhu, X.; Russell, T. P. J. Mater. Chem. A 2016, 4, 17354; (c) Sun, Y. M.; Seifter, J.; Huo, L. J.; Yang, Y. L.; Hsu, B. B. Y.; Zhou, H. Q.; Sun, X. B.; Xiao, S.; Jiang, L.; Heeger, A. J. Adv. Energy Mater. 2014, 1400987.
-
[5]
(a) Anthony, J. E. Chem. Mater. 2011, 23, 583; (b) Brunetti, F. G.; Gong, X.; Tong, M.; Heeger, A. J.; Wudl, F. Angew. Chem. Int. Ed. 2010, 49, 532; (c) Gao, G.; Zhang, X.; Meng, D.; Zhang, A.; Liu, Y.; Jiang, W.; Sun, Y.; Wang, Z. RSC Adv. 2016, 6, 14027; (d) Liu, T.; Guo, Y.; Yi, Y.; Huo, L.; Xue, X.; Sun, X.; Fu, H.; Xiong, W.; Meng, D.; Wang, Z.; Liu, F.; Russell, T. P.; Sun, Y. Adv. Mater. 2016, 28, 10008; (e) Meng, D.; Sun, D.; Zhong, C.; Liu, T.; Fan, B.; Huo, L.; Li, Y.; Jiang, W.; Choi, H.; Kim, T.; Kim, J. Y.; Sun, Y.; Heeger, A. J. J. Am. Chem. Soc. 2016, 138, 375.
-
[6]
(a) Zhou, Y.; Kurosawa, T.; Ma, W.; Guo, Y.; Fang, L.; Vandewal, K.; Diao, Y.; Wang, C.; Yan, Q.; Reinspach, J.; Mei, J.; Appleton, A.; Koleilat, G. I.; Gao, Y.; Mannsfeld, S. C. B.; Salleo, A.; Ade, H.; Zhao, D.; Bao, Z. Adv. Mater. 2014, 26, 3767; (b) Li, Z.; Xu, X.; Zhang, W.; Meng, X.; Ma, W.; Yartsev, A.; Inganäs, O.; Andersson, M. R.; Janssen, R. A. J.; Wang, E. J. Am. Chem. Soc. 2016, 138, 10935.
-
[7]
(a) Lin, Y.; Zhao, F.; Wu, Y.; Chen, K.; Xia, Y.; Li, G.; Prasad, S. K. K.; Zhu, J.; Huo, L.; Bin, H.; Zhang, Z. G.; Guo, X.; Zhang, M.; Sun, Y.; Gao, F.; Wei, Z.; Ma, W.; Wang, C.; Hodgkiss, J.; Bo, Z.; Inganäs, O.; Li, Y.; Zhan, X. Adv. Mater. 2017, 29, 1604155; (b) Zhao, J.; Li, Y.; Yang, G.; Jiang, K.; Lin, H.; Ade, H.; Ma, W.; Yan, H. Nat. Energy 2016, 1, 15027; (c) He, Z.; Xiao, B.; Liu, F.; Wu, H.; Yang, Y.; Xiao, S.; Wang, C.; Russell, T. P.; Cao, Y. Nat. Photon. 2015, 9, 174; (d) Li, S.; Ye, L.; Zhao, W.; Zhang, S.; Mukherjee, S.; Ade, H.; Hou, J. Adv. Mater. 2016, 28, 9423; (e) Zhao, W.; Li, S.; Zhang, S.; Liu, X.; Hou, J. Adv Mater. 2017, 29, 1604059; (f) Yang, Y.; Zhang, Z. G.; Bin, H.; Chen, S.; Gao, L.; Xue, L.; Yang, C.; Li, Y. J. Am. Chem. Soc. 2016, 138, 15011.
-
[8]
Nguyen, D. T. T.; Kim, T.; Li, Y.; Song, S.; Nguyen, T. L.; Uddin, M. A.; Hwang, S.; Kim, J. Y.; Woo, H. Y. J. Polym. Sci, Part A: Polym. Chem. 2016, 54, 3826. doi: 10.1002/pola.v54.24
-
[9]
(a) Liu, Y.; Zhao, J.; Li, Z.; Mu, C.; Ma, W.; Hu, H.; Jiang, K.; Lin, H.; Ade, H.; Yan, H. Nat. Commun. 2014, 5, 5293; (b) Zhang, S.; Yang, B.; Liu, D.; Zhang, H.; Zhao, W.; Wang, Q.; He, C.; Hou, J. Macromolecules 2016, 49, 120; (c) Stuart, A. C.; Tumbleston, J. R.; Zhou, H.; Li, W.; Liu, S.; Ade, H.; You, W. J. Am. Chem. Soc. 2013, 135, 1806; (d) Hu, H.; Jiang, K.; Yang, G.; Liu, J.; Li, Z.; Lin, H.; Liu, Y.; Zhao, J.; Zhang, J.; Huang, F.; Qu, Y.; Ma, W.; Yan, H. J. Am. Chem. Soc. 2015, 137, 14149; (e) Tang, D.; Liu, Y.; Zhang, Z.; Shu, Q.; Wang, B.; Fan, J.; Song, B. Org. Electron. 2016, 33, 187.
-
[10]
(a) Choi, H. ; Ko, S. J. ; Kim, T. ; Morin, P. O. ; Walker, B. ; Lee, B. H. ; Leclerc, M. ; Kim, J. Y. ; Heeger, A. J. Adv. Mater. 2015, 27, 3318; (b) Zhao, C. B. ; Wang, Z. L. ; Zhou, K. ; Ge, H. G. ; Zhang, Q. ; Jin, L. X. ; Wang, W. L. ; Yin, S. W. Acta Chim. Sinica 2016, 74, 251 (in Chinese). (赵蔡斌, 王占领, 周科, 葛红光, 张强, 靳玲侠, 王文亮, 尹世伟, 化学学报, 2016, 74, 251. )
-
[11]
Bin, H.; Zhang, Z. G.; Gao, L.; Chen, S.; Zhong, L.; Xue, L.; Yang, C.; Li, Y. J. Am. Chem. Soc. 2016, 138, 4657. doi: 10.1021/jacs.6b01744
-
[12]
(a) Chen, H. Y.; Hou, J.; Zhang, S.; Liang, Y.; Yang, G.; Yang, Y.; Yu, L.; Wu, Y.; Li, G. Nat. Photonics 2009, 3, 649; (b) Lu, L.; Yu, L. Adv. Mater. 2014, 26, 4413; (c) Liang, Y.; Xu, Z.; Xia, J.; Tsai, S. T.; Wu, Y.; Li, G.; Ray, C.; Yu, L. Adv. Mater. 2010, 22, E135.
-
[13]
(a) Lee, W. ; Kim, G. H. ; Ko, S. J. ; Yum, S. ; Hwang, S. ; Cho, S. ; Shin, Y. H. ; Kim, J. Y. ; Woo, H. Y. Macromolecules 2014, 47, 1604; (b) Qin, R. ; Li, W. ; Li, C. ; Du, C. ; Veit, C. ; Schleiermacher, H. F. ; Andersson, M. ; Bo, Z. ; Liu, Z. ; Inganäs, O. ; Wuerfel, U. ; Zhang, F. J. Am. Chem. Soc. 2009, 131, 14612; (c) Zhang, C. ; Sun, Y. ; Dai, B. ; Zhang, X. ; Yang, H. ; Lin, B. ; Guo, L. Chin. J. Org. Chem. 2014, 34, 1701 (in Chinese). (张超, 孙莹, 戴斌, 张雪勤, 杨洪, 林保平, 郭玲香, 有机化学, 2014, 34, 1701. ); (d) Liu, Z. ; Xu, F. ; Yan, D. Acta Chim. Sinica. 2014, 72, 171 (in Chinese). (刘震, 徐丰, 严大东, 化学学报, 2014, 72, 171. ); (e) Ye, H. ; Li, W. ; Li, W. Chin. J. Org. Chem. 2012, 32, 266 (in Chinese). (叶怀英, 李文, 李维实, 有机化学, 2012, 32, 266. )
-
[14]
(a) Reichenbächer, K.; Süss, H. I.; Hulliger, J. Chem. Soc. Rev. 2005, 34, 22; (b) Lee, W.; Choi, H.; Hwang, S.; Kim, J. Y.; Woo, H. Y. Chem. Eur. J. 2012, 18, 2551; (c) Li, Y.; Lee, T. H.; Park, S. Y.; Uddin, M. A.; Kim, T.; Hwang, S.; Kim, J. Y.; Woo, H. Y. Polym. Chem. 2016, 7, 463; (d) Yao, H.; Yu, R.; Shin, T. J.; Zhang, H.; Zhang, S.; Jang, B.; Uddin, M. A.; Woo, H. Y.; Hou, J. Adv. Energy Mater. 2016, 6, 1600742; (e) Seifter, J.; Sun, Y.; Choi, H.; Lee, B. H.; Nguyen, T. L.; Woo, H. Y.; Heeger A. J. Adv. Mater. 2015, 27, 4989; (f) Gallaher, J. K.; Prasad, S. K. K.; Uddin, M. A.; Kim, T.; Kim, J. Y.; Woo, H. Y.; Hodgkiss, J. M. Energy Environ. Sci. 2015, 8, 2713.
-
[15]
Nguyen, T. L.; Choi, H.; Ko, S. J.; Uddin, M. A.; Walker, B.; Yum, S.; Jeong, J. E.; Yun, M. H.; Shin, T. J.; Hwang, S.; Kim, J. Y.; Woo, H. Y. Energy Environ. Sci. 2014, 7, 3040. doi: 10.1039/C4EE01529K
-
[16]
Patra, A.; Bendikov, M. J. Mater. Chem. 2010, 20, 422. doi: 10.1039/B908983G
-
[17]
(a) Lee, J.; Han, A. R.; Kim, J.; Kim, Y.; Oh, J. H.; Yang, C. J. Am. Chem. Soc. 2012, 134, 20713; (b) Kronemeijer, A. J.; Gili, E.; Shahid, M.; Rivnay, J.; Salleo, A.; Heeney, M.; Sirringhaus, H. Adv. Mater. 2012, 24, 1558; (c) Alghamdi, A. A. B.; Watters, D. C.; Yi, H.; Al-Faifi, S.; Almeataq, M. S.; Coles, D.; Kingsley, J.; Lidzey, D. G.; Iraqi, A. J. Mater. Chem. A 2013, 1, 5165.
-
[18]
Scharber, M. C.; Mühlbacher, D.; Koppe, M.; Denk, P.; Waldauf, C.; Heeger, A. J.; Brabec, C. J. Adv. Mater. 2006, 18, 789. doi: 10.1002/(ISSN)1521-4095
-
[19]
(a) Chu, T. Y.; Lu, J.; Beaupré, S.; Zhang, Y.; Pouliot, J. R.; Zhou, J.; Najari, A.; Leclerc, M.; Tao, Y. Adv. Funct. Mater. 2012, 22, 2345; (b) Kang, H.; Uddin, M. A.; Lee, C.; Kim, K. H.; Nguyen, T. L.; Lee, W.; Li, Y.; Wang, C.; Woo, H. Y.; Kim, B. J. J. Am. Chem. Soc. 2015, 137, 2359.
-
[20]
Yu, D.; Oyewole, O. K.; Kwabi, D.; Tong, T.; Anye, V. C.; Asare, J.; Rwenyagila, E.; Fashina, A.; Akogwu, O.; Du, J.; Soboyejo, W. O. J. Appl. Phys. 2014, 116, 074506. doi: 10.1063/1.4892393
-
[21]
(a) Yao, E. P.; Chen, C. C.; Gao, J.; Liu, Y.; Chen, Q.; Cai, M.; Hsu, W. C.; Hong, Z.; Li, G.; Yang, Y. Sol. Energy Mater. Sol. Cells 2014, 130, 20; (b) Huang, T. Y.; Patra, D.; Hsiao, Y. S.; Chang, S. H.; Wu, C. G.; Ho, K. C.; Chu, C. W. J. Mater. Chem. A 2015, 3, 10512.
-
[22]
(a) Kim, Y.; Yeom, H. R.; Kim, J. Y.; Yang, C. Energy Environ. Sci. 2013, 6, 1909; (b) Proctor, C. M.; Kuika, M.; Nguyena, T. Q. Prog. Polym. Sci. 2013, 38, 1941.
-
[23]
(a) Zhang, H.; Li, S.; Xu, B.; Yao, H.; Yang, B.; Hou, J. J. Mater. Chem. A 2016, 4, 18043; (b) Cowan, S. R.; Roy, A.; Heeger, A. J. Phys. Rev. B 2010, 82, 245207.
-
[24]
Bull, S. R.; Palmer, L. C.; Fry, N. J.; Greenfield, M. A.; Messmore, B. W.; Meade, T. J.; Stupp, S. I. J. Am. Chem. Soc. 2008, 130, 2742. doi: 10.1021/ja710749q
-
[25]
Zhang, W.; Zhang, F.; Tang, R.; Fu, Y.; Wang, X.; Zhuang, X.; He, G.; Feng, X. Org. Lett. 2016, 18, 3618. doi: 10.1021/acs.orglett.6b01659
-
[26]
Uy, R. L.; Yan, L.; Li, W.; You, W. Macromolecules 2014, 47, 2289. doi: 10.1021/ma5001095
-
[1]
-
Table 1. Properties of PBT2F-TT-a, PBT2F-TT and PBT2F-Se
Polymer ΔT5%/℃ λsolutiona/nm λfilm/nm λonsetb/nm Egc/eV HOMOd/eV LUMOe/eV PBT2F-TT-a 339 650, 598, 439 658, 600, 446 716 1.73 -5.40 -3.67 PBT2F-TT 340 661, 600, 443 662, 603, 447 716 1.73 -5.35 -3.62 PBT2F-Se 292 686, 630, 438 700, 631, 443 738 1.68 -5.37 -3.69 a Measured in dilute o-dichlorobenzene at room temperature. b Measured in the thin film. c Calculated from the absorption edge of the thin film. d Determined from cyclic voltammetry measurement (on conductive ITO substrate). e LUMO=HOMO+Eg. Table 2. Optimized photovoltaic properties of the PSCs based on different polymers/PC71BM blends under the illumination of AM1.5G, 100 mW/cm2
Polymera DPE additive VOC/V JSC/(mA•cm-2) FF/% PCEmax/% PCEave/% PBT2F-TT-a - 0.74 7.32 56.1 3.10 3.06 PBT2F-TT-a 2% 0.73 8.56 55.5 3.48 3.41 PBT2F-TT - 0.71 9.50 56.3 3.82 3.80 PBT2F-TT 2% 0.70 10.44 57.2 4.22 4.15 PBT2F-Se - 0.78 7.42 51.5 3.01 2.94 PBT2F-Se 2% 0.72 10.75 53.7 4.18 4.15 aThe polymer:PC71BM weight ratios were 1.5:1 (w/w), 1:1 (w/w) and 1:1 (w/w) for PBT2F-TT-a, PBT2F-TT and PBT2F-Se, respectively. Table 3. The parameters of the equivalent circuits for the PSCs operated in the dark near the corresponding VOC bias voltages
Polymer DPE additive Rs/(Ω•cm2) R1/(Ω•cm2) C1/(F•cm-2) R2/(Ω•cm2) C2/(F•cm-2) PBT2F-TT-a — 1.08 5.86 1.63×10-10 26.01 2.23×10-10 PBT2F-TT-a 2% 1.05 5.76 2.21×10-10 13.54 3.82×10-10 PBT2F-TT — 1.01 5.63 1.48×10-10 10.13 2.99×10-10 PBT2F-TT 2% 1.15 2.80 2.77×10-10 8.67 3.85×10-10 PBT2F-Se — 1.07 3.16 2.70×10-10 20.72 2.29×10-10 PBT2F-Se 2% 1.12 1.78 3.29×10-10 5.54 3.59×10-10 Table 4. SCLC mobilities of the devices
Polymer DPE additive Hole μ/(cm2•V-1•s-1) Electron μ/(cm2•V-1•s-1) PBT2F-TT-a — 1.53×10-5 2.12×10-4 PBT2F-TT-a 2% 2.13×10-5 2.72×10-4 PBT2F-TT — 1.30×10-5 6.46×10-6 PBT2F-TT 2% 1.34×10-5 9.72×10-6 PBT2F-Se — 4.86×10-6 8.12×10-5 PBT2F-Se 2% 8.86×10-6 8.22×10-5 -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 8
- 文章访问数: 804
- HTML全文浏览量: 55



 下载:
下载:





 下载:
下载:

