
基于轴手性配体的两个镧系金属配合物的合成、表征及其荧光性质
English
Syntheses, Characterization and Fluorescence Properties Analysis of Two Lanthanide Coordination Polymers Based on Axial Chirality Ligand 2, 2'-Dinitro-4, 4'-biphenyldicarboxylic Acid
-
Key words:
- coordination polymer
- / hydrothermal condition
- / fluorescence
- / chirality
-
Coordination polymers have received attention because of the intriguing structures and potential applications in magnetism, catalysis, luminescence, conductivity, sensors, porosity, nonlinear optics, etc[1-18]. Luminescent lanthanide complexes have been extensively studied due to extremely narrow emission bands, large Stokes shift, long luminescence decay times, and no theoretical cap on quantum efficiency; particularly, europium, terbium, samarium, and dysprosium have interesting luminescent properties, such as red emission for Eu (Ⅲ) ion. They are very attractive for applications in light-emitting diodes[19], laser materials[20], and as probes and labels in a variety of biological and chemical applications[21-22]. Luminescent intensities of lanthanide complexes are dependent on the ligands. In our continuing effort in this field[23-24], we chose ligand 2, 2′-dinitro-4, 4′-biphenyldicarboxylic acid (H2nbpdc) as an organic building block in an attempt to construct coordination polymers with new structural features. In addition, the two benzoate moieties in nbpdc twist freely due to two nitro groups, thus bringing about an organic building block which is geometrically different from the mother ligand and hence may lead to different topologies. Moreover, nbpdc ligand may induce cluster aggrega-tion and link the clusters into extended networks. However, to the best of our knowledge, frameworks based on ligand nbpdc have rarely been studied[25]. Herein, we report the synthesis, crystal structure, and luminescent properties of two lanthanide (Ⅲ) complexes.
1 Experimental
1.1 Materials and physical measurements
All of the chemicals and solvents except for nbpdc ligand in this study obtained from Shanghai Jingchun Scientific Company were of reagent grade and used without any further purification. The nbpdc ligand was prepared by the method reported previou-sly[26]. Infrared spectra were recorded on a SHIMADZU IR prestige-21 FTIR-8400S spectrometer in the spectral range of 4000~500 cm-1 with samples in the form of potassium bromide pellets. Elemental analyses were taken on a Perkin-Elmer 240C elemental analyzer. The solid-state fluorescent spectra were recorded by Spectrofluorometer FS5 at room temperature. Thermo-gravimetric analyses (TGA) were conducted on a NETZSCH TG 209 F3 thermo gravimeter with the heating rate of 10 K·min-1 in a N2 atmosphere. The chirality of complexes was characterized by JASCO J-1500 circular dichroism.
1.2 Preparation of complex 1
H2nbpdc (0.1 mmol, 0.033 0 g), phen (0.1 mmol, 0.019 8 g) and La (NO3)3 (0.1 mmol, 0.043 3 g) were placed in a 25 mL Teflon-lined stainless-steel vessel. After the addition of water (1.5 mL) and DMF (1.5 mL), the solution was heated to 125 ℃ for three days. Afterwards, the reaction system was slowly cooled down (a descent rate of 5 ℃·h-1) to room temperature. Colorless transparent bulk crystals were obtained, washed with distilled water, and dried in air. Yield: 64% (based on nbpdc). Anal. Calcd.(%) for C27H14N4 O10La: C, 46.73; H, 2.01; N, 8.08. Found (%): C, 45.47; H, 1.96; N, 7.39. IR data (KBr pellet, cm-1) (Fig.S1): 3 402(w), 3 090(w), 2 922(w), 2 838(w), 1 599(s), 1 515(m), 1 396(s), 1 335(s), 1 094(w), 913(w), 842(m), 770(s), 710(m), 517(w).
1.3 Preparation of complex 2
The synthesis of 2 was similar with 1 except that Eu (NO3)3 (0.1 mmol, 0.044 6 g) was used instead of La (NO3)3. Colorless block crystals were obtained in 52% yield. Anal. Calcd.(%) for C27H15N4O10Eu: C, 45.80; H, 2.12; N, 7.92. Found (%): C, 44.93; H, 2.03; N, 6.46. IR data (KBr pellet, cm-1) (Fig.S2): 3 402(s), 3 086(m), 2 948(w), 2 855(w), 1 652(w), 1 596(w), 1 523(s), 1 403(s), 1 338(m), 1 078(m), 995(w), 912(m), 839(s), 773(s), 719(s), 624(m).
1.4 Single-crystal X-ray diffraction
Single crystals of 1 and 2 with appropriate dime-nsions (0.26 mm×0.30 mm×0.24 mm for 1, 0.30 mm×0.30 mm×0.20 mm for 2) were selected for data colle-ction on a Rigaku SCXmini diffractometer equipped with a graphite-monochromated Mo Kα radiation (λ=0.071 073 nm), using an ω scan mode at 296 K. The absorption correction was carried out by a multi-scan method. The structures were solved by direct methods with SHELXS-97 and refined by full-matrix least-squares on F2 with SHELXL-97[27-28]. While H atoms were located in calculated positions and refined using a riding model, which were found by electron cloud density. Some disordered solvent molecules were treated by SQUEEZE[29] process. Topology information for complexes 1~2 was obtained using TOPOS 4.0[30]. All non-hydrogen atoms were refined with anisotropic thermal parameters. All hydrogen atoms attached to C, N and O atoms were added theoretically and refined with a riding model and fixed isotropic thermal parameters. Detailed data collection and refinement of complexes 1 and 2 are summarized in Table 1, and the selected bond distances and bond angles of 1 and 2 are listed in Table 2 and 3, respectively.
1 2 Formula C27H14N4O10La dNAoEu Formula weight 693.33 707.39 Crystal system Orthorhombic Orthorhombic Space group P212121 P212121 a / nm 0.855 20(9) 0.848 78(5) b / nm 1.096 95(10) 1.086 70(5) c / nm 2.727 4(2) 2.690 14(13) V / nm3 2.558 7(4) 2.481 3(2) Z 4 4 Dc / (g·cm-3) 1.800 1.894 μ/ mm-1 1.739 2.599 R1, wR2 (I > 2σ-(I)) 0.024 3, 0.056 7 0.028 3, 0.070 7 R1, wR2 (all data) 0.030 3, 0.075 6 0.030 4, 0.084 2 Flack 0.000(15) 0.005(13) Table 1. Data collection and processing parameters for 1 and 2La (1)-N (1) 0.275 7(3) La (1)-O (2) 0.245 7(3) La (1)-O (4) 0.253 9(3) La (1)-O (4) 0.281 2(3) La (1)-O (6) 0.247 6(3) La (1)-O (7) 0.250 0(3) La (1)-O (1) 0.248 7(3) La (1)-N (3) 0.276 7(4) La (1)-C (4) 0.302 9(4) La (1)-O (3) 0.255 1(3) N (1)-La (1)-O (4) 110.65(10) N (1)-La (1)-N (3) 59.43(11) N (1)-La (1)-C (4) 91.16(11) O (2)-La (1)-N (1) 141.66(10) O (2)-La (1)-O (4) 71.26(9) O (2)-La (1)-O (4) 65.77(9) O (2)-La (1)-O (6) 74.90(10) O (2)-La (1)-O (7) 88.94(10) O (2)-La (1)-O (1) 133.84(10) O (2)-La (1)-N (3) 146.21(11) O (2)-La (1)-C (4) 71.67(10) O (2)-La (1)-O (3) 77.85(10) O (4)-La (1)-N (1) 119.20(10) O (4)-La (1)-O (4) 129.72(3) O (4)-La (1)-N (3) 74.95(10) O (4)-La (1)-C (4) 142.92(10) O (4)-La (1)-C (4) 24.64(9) O (4)-La (1)-O (3) 141.96(10) O (6)-La (1)-N (1) 72.70(10) O (6)-La (1)-O (4) 114.40(9) O (6)-La (1)-O (4) 76.51(10) O (6)-La (1)-O (7) 147.86(10) O (6)-La (1)-O (1) 137.32(10) O (6)-La (1)-N (3) 97.85(11) O (6)-La (1)-C (4) 94.86(10) O (6)-La (1)-O (3) 74.41(10) O (7)-La (1)-N (1) 129.22(10) O (7)-La (1)-O (4) 71.99(10) O (7)-La (1)-O (4) 82.18(9) O (7)-La (1)-N (3) 80.18(11) O (7)-La (1)-C (4) 106.35(10) O (7)-La (1)-O (3) 129.78(10) O (1)-La (1)-N (1) 66.90(10) O (1)-La (1)-O (4) 69.75(9) O (1)-La (1)-O (4) 135.96(10) O (1)-La (1)-O (7) 73.22(10) O (1)-La (1)-N (3) 73.24(11) O (1)-La (1)-C (4) 73.24(10) O (1)-La (1)-O (3) 81.83(10) N (3)-La (1)-O (4) 142.22(10) N (3)-La (1)-C (4) 142.08(11) O (3)-La (1)-N (1) 74.19(10) O (3)-La (1)-O (4) 48.11(9) O (3)-La (1)-N (3) 132.86(10) O (3)-La (1)-C (4) 23.55(10) Table 2. Selected bond lengths (nm) and bond angles (°) of 1Eu (1)-O (2) 0.236 9(4) Eu (1)-O (7) 0.238 1(3) Eu (1)-O (1) 0.238 4(3) Eu (1)-O (8) 0.239 8(4) Eu (1)-O (10) 0.242 0(3) Eu (1)-O (9) 0.242 3(3) Eu (1)-N (2) 0.266 5(4) Eu (1)-N (1) 0.268 0(5) Eu (1)-O (10) 0.286 2(3) O (2)-Eu (1)-O (7) 75.72(13) O (2)-Eu (1)-O (1) 132.07(12) O (7)-Eu (1)-O (1) 137.28(14) O (2)-Eu (1)-O (8) 86.29(13) O (7)-Eu (1)-O (8) 147.85(13) O (1)-Eu (1)-O (8) 74.03(13) O (2)-Eu (1)-O (10) 71.93(12) O (7)-Eu (1)-O (10) 75.94(13) O (1)-Eu (1)-O (10) 137.13(13) O (8)-Eu (1)-O (10) 73.20(12) O (2)-Eu (1)-O (9) 77.57(13) O (7)-Eu (1)-O (9) 75.38(11) O (1)-Eu (1)-O (9) 80.32(12) O (8)-Eu (1)-O (9) 126.85(12) O (10)-Eu (1)-O (9) 142.24(12) O (2)-Eu (1)-N (2) 146.06(13) O (7)-Eu (1)-N (2) 98.23(13) O (1)-Eu (1)-N (2) 74.52(12) O (8)-Eu (1)-N (2) 81.94(15) O (10)-Eu (1)-N (2) 74.20(13) O (9)-Eu (1)-N (2) 134.03(14) O (1)-Eu (1)-N (2) 74.52(12) O (1)-Eu (1)-N (2) 74.52(12) O (1)-Eu (1)-N (2) 74.52(12) O (2)-Eu (1)-N (1) 141.14(13) O (7)-Eu (1)-N (1) 72.13(13) O (1)-Eu (1)-N (1) 67.55(12) O (8)-Eu (1)-N (1) 132.10(13) O (10)-Eu (1)-N (1) 119.25(12) O (9)-Eu (1)-N (1) 73.69(13) N (2)-Eu (1)-N (1) 61.34(14) O (2)-Eu (1)-O (10) 65.16(11) O (7)-Eu (1)-O (10) 115.27(11) O (1)-Eu (1)-O (10) 68.37(11) O (8)-Eu (1)-O (10) 79.17(12) O (10)-Eu (1)-O (10) 129.82(4) O (9)-Eu (1)-O (10) 48.02(11) N (2)-Eu (1)-O (10) 141.70(12) N (1)-Eu (1)-O (10) 110.44(12) Table 3. Selected bond lengths (nm) and bond angles (°) of 2CCDC: 1504875, 1; 1504877, 2.
1.5 Dielectric measurements
The temperature dependences of the dielectric constants of two complexes were measured on the Tonghui TH2828A analyzer in the temperature ranges of 120~290 K and 150~230 K, respectively, within the frequency range of 500 Hz to 1 MHz at the AC voltage of 1 V. Crystals of the two compounds with appropriate dimensions were prepared with silver-conductive glue and used for dielectric studies.
2 Results and discussion
2.1 Crystal structures of 1 and 2
Single-crystal X-ray diffraction reveals that complexes 1 and 2 crystallize in the orthorhombic system, space group P212121 and displays a 3D coor-dination framework. Complexes 1 and 2 are isostru-ctural, so the crystal structure of 1 is described in detail. As shown in Fig. 1a, the asymmetric unit of crystal 1 contains one La (Ⅲ) ion, one nbpdc ligand, one phen and one formic acid ion (FA). Besides, the La (Ⅲ) is in a slightly distorted dodecahedral environ-ment. Moreover, it is interesting that only ligand nbpdc coordinates metal La (Ⅲ) ion as a two-dimensional network that gradually forms a three-dimensional structure by coordinating phen and La (Ⅲ) ions (Fig. 1b). To get a better insight into the structure of complex 1, topological analysis was conducted. Each 2-connected bridging nbpdc ligand can be regarded as a linear linker. Each phen ligand can also be treated as a 2-connector and each La (Ⅲ) ion can be regarded as 8-connected node since it links four nbpdc, one phen and two formic acid ions. Thus, the simplified overall structure of 1 is 4-connected uninodal net with stoich-iometry (4-c), as shown in Fig. 1c. The point (Schläfli) symbol for net is (44, 62) calculated by TOPOS program and the topological type is sql.
2.2 Thermogravimetric analysis
In order to investigate the thermal stability of complexes 1~2, TGA was performed in N2 atmosphere. As shown in Fig.S3, the TGA curve of complex 1 display three weight loss steps. The first weight loss from 480 to 590 ℃ is attributed to the loss of one formic acid ion (Obsd. 6.7%, Calcd. 6.3%). The second weight loss of 1 between 600 and 860 ℃ perhaps results from the release of coordinated 1, 10-Phenanthroline molecule (Obsd. 26.5%, Calcd. 25.9%). Upon heating, the framework decomposed at above 900 ℃. For the complex 2, the data display the first weight loss platform at above 500 ℃, corresponding to the release of one formic acid ion (Obsd. 6.5%, Calcd. 6.2%). Upon temperature increase, the structure began to collapse slowly.
2.3 Luminescent properties
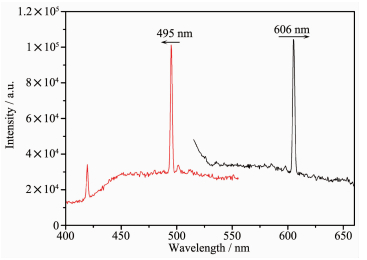
The luminescent properties of 1 and 2 in the solid state were investigated at room temperature. As shown in Fig. 2, complex 1 exhibits maximal emission peaks at 606 nm (λex=495 nm), suggesting that 1 may be a good visible light-emitting material. The photolu-minescent mechanism is tentatively attributed to ligand-to-ligand transitions, being in reasonable agreement with this kind of metal complexes.
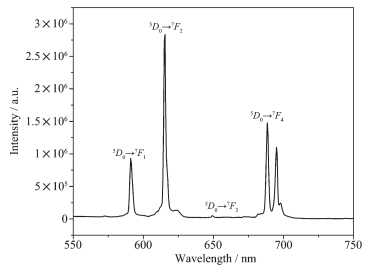
The fluorescence spectrum of 2 was evaluated in the solid state upon the excitation at 395.5 nm at room temperature showing the 5D0→7F0 (J=0~4) chara-cteristic transitions of Eu (Ⅲ). The strongest narrow-band emission at 615.5 nm is characteristic of the hypersensitive 5D0→7F1 transition of Eu (Ⅲ), which is more intense than the 5D0→7F0 transition at 591 nm, 5D0→7F3 transition at 688 nm and 5D0→7F4 transition at 697 nm. One weak emission bands at 649 nm correspond to 5D0→7F2 transitions (Fig. 3). Intense red fluorescence for 2 is observed under UV excitation, suggesting that it has potential application in photoactive materials.
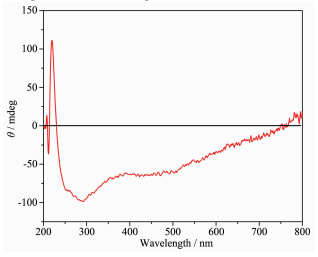
2.4 Circular dichroism (CD) analysis
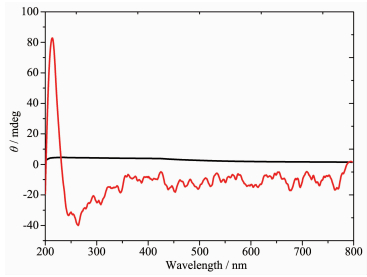
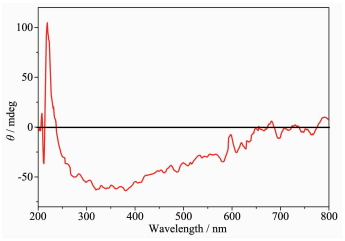
According to the P212121 space group of complexes 1 and 2 and a chirality of H2nbpdc ligand (Fig. 4), the study on the chirality of complexes 1 and 2 is conducted, respectively. As shown in Fig. 5~6, compared with H2nbpdc, complexes 1 and 2 have appeared correspondingly higher positive peaks at a wavelength of about 220 nm, which can be regarded as the peak of the chiral ligands. In addition, the ligand appeared wide peak at 289 nm, and compared to complexes 1 and 2, it is expected that the peak of the complexes are similar to the peak of ligand. So it is further proved that the complexes have the chirality.
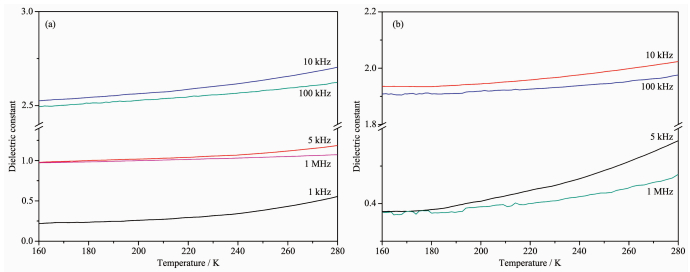
2.5 Dielectric properties
Materials with temperature-dependent dielectric constants which rapidly fluctuate may have phase transition, piezoelectric, and ferroelectric properties. The permitivities (ε=ε1-iε2) of powdered samples of the two complexes as pellets were measured, where ε1 and ε2 are the corresponding real part and imaginary part of dielectric constant, respectively.
In addition, the dielectric constants of two complexes show similar temperature dependence (Fig. 7). The temperature-dependence dielectric constant at different frequency indicated that the dielectric constants (ε1) gradually increase with a rise of temper-ature (160~280 K).
3 Conclusions
Two metal-organic coordination polymers based on 2, 2′-dinitro-4, 4′-biphenyldicarboxylic acid were successfully synthesized under hydrothermal conditions and structurally characterized. Complexes 1 and 2 generated a 3D framework with (44, 62) topologies. Furthermore, complexes 1 and 2 exhibited strong fluorescent emission in the solid state at room temperature.
Supporting information is available at http://www.wjhxxb.cn
-
-
[1]
Delgado S, Sanz miguel P J, Priego J L, et al. Inorg. Chem., 2008, 47:9128-9130 doi: 10.1021/ic801314s
-
[2]
Thallapally P K, Tian J, Motkuri R K, et al. J. Am. Chem. Soc., 2008, 130:16842-16843 doi: 10.1021/ja806391k
-
[3]
Wang P, Ma J P, Dong Y B, et al. J. Am. Chem. Soc., 2007, 129:10620-10621 doi: 10.1021/ja072442h
-
[4]
Cai S L, Zheng S R, Fan J, et al. J. Solid State Chem., 2011, 184:3172-3178 doi: 10.1016/j.jssc.2011.09.006
-
[5]
Hu S, He K H, Zeng M H, et al. Inorg. Chem., 2008, 47:5218-5224 doi: 10.1021/ic800050u
-
[6]
Eddaoudi M, Moler D B, Li H, et al. Acc. Chem. Res., 2001, 43:319-330
-
[7]
Evans O R, Lin W. Acc. Chem. Res., 2002, 35:511-522 doi: 10.1021/ar0001012
-
[8]
Rosi N L, Kim J, Eddaoudi M, et al. J. Am. Chem. Soc., 2005, 127:1504-1518 doi: 10.1021/ja045123o
-
[9]
Chen L Z, Wang F M, Shu H. J. Coord. Chem., 2012, 65:439-452 doi: 10.1080/00958972.2012.654786
-
[10]
Farha O K, Hupp J T. Acc. Chem. Res., 2010, 43:1166-1175 doi: 10.1021/ar1000617
-
[11]
Peng R, Li M, Li D. Coord. Chem. Rev., 2010, 254:1-18 doi: 10.1016/j.ccr.2009.10.003
-
[12]
Zhou H C, Long J, Yaghi O M. Chem. Rev., 2012, 112:673-674 doi: 10.1021/cr300014x
-
[13]
Zhao H, Qu Z R, Ye H Y, et al. Chem. Soc. Rev., 2008, 37:84-100 doi: 10.1039/B616738C
-
[14]
Xu G, Guo G C, Wang M S, et al. Angew. Chem. Int. Ed., 2007, 46:3249-3251 doi: 10.1002/(ISSN)1521-3773
-
[15]
Zhang W, Xiong R G. Chem. Rev., 2012, 112:1163-1195 doi: 10.1021/cr200174w
-
[16]
Xu G C, Zhang W, Ma X M, et al. J. Am. Chem. Soc., 2011, 113:14948-14951
-
[17]
陈立庄, 曹星星, 汪芳明, 等.无机化学学报, 2012, 28(6):1291-1297 http://www.cnki.com.cn/Article/CJFDTOTAL-SYQY201603027.htmCHEN Li-Zhuang, CAO Xing-Xing, WANG Fang-Ming, et al. Chinese J. Inorg. Chem., 2012, 28(6):1291-1297 http://www.cnki.com.cn/Article/CJFDTOTAL-SYQY201603027.htm
-
[18]
黄登登, 陈立庄.无机化学学报, 2015, 31(2):377-384 http://www.cnki.com.cn/Article/CJFDTOTAL-SYQY201603027.htmHUANG Deng-Deng, CHEN Li-Zhuang. Chinese J. Inorg. Chem., 2015, 31(2):377-384 http://www.cnki.com.cn/Article/CJFDTOTAL-SYQY201603027.htm
-
[19]
McGehee M D, Bergstedt T, Zhang C, et al. Adv. Mater., 1999, 11:1349-13549 doi: 10.1002/(ISSN)1521-4095
-
[20]
Piguet C, Bunzli J C G, Bernardinelli G, et al. J. Am. Chem. Soc., 1993, 115:8197-8206 doi: 10.1021/ja00071a032
-
[21]
Zhao B, Chen X Y, Chen P, et al. J. Am. Chem. Soc., 2004, 126:15394-15395 doi: 10.1021/ja047141b
-
[22]
Sun L N, Zhang H J, Meng Q G, et al. J. Phys. Chem. B, 2005, 109:6174-6182 http://www.ncbi.nlm.nih.gov/pubmed/16851683
-
[23]
Chen L Z, Huang Y, Xiong R G, et al. J. Mol. Struct., 2010, 963:16-21 doi: 10.1016/j.molstruc.2009.09.038
-
[24]
Chen L Z, Huang D D. Chin. Chem. Lett., 2014, 25:279-282 doi: 10.1016/j.cclet.2013.11.031
-
[25]
Chen L Z, Pan Q J, Cao X X, et al. CrystEngComm, 2016, 18:1944-1952 doi: 10.1039/C5CE02426A
-
[26]
Wu R F, Zhang T L, Qiao X J. Chin. Chem. Lett., 2010, 21:1007-1010 doi: 10.1016/j.cclet.2010.02.006
-
[27]
Sheldrick G M. SHELXS-97, Program for Crystal Structure Solution, University of Göttingen, Germany, 1997.
-
[28]
Sheldrick G M. SHELXL-97, Program for Crystal Structure Refinement, University of Göttingen, Germany, 1997.
-
[29]
Spek A L. Acta Crystallogr. Sect. C, 2015, C71:9-18
-
[30]
Blatov V, Shevchenko A, Serezhkin V, et al. J. Appl. Crystallogr., 2000, 33:1193 doi: 10.1107/S0021889800007202
-
[1]
-
Table 1. Data collection and processing parameters for 1 and 2
1 2 Formula C27H14N4O10La dNAoEu Formula weight 693.33 707.39 Crystal system Orthorhombic Orthorhombic Space group P212121 P212121 a / nm 0.855 20(9) 0.848 78(5) b / nm 1.096 95(10) 1.086 70(5) c / nm 2.727 4(2) 2.690 14(13) V / nm3 2.558 7(4) 2.481 3(2) Z 4 4 Dc / (g·cm-3) 1.800 1.894 μ/ mm-1 1.739 2.599 R1, wR2 (I > 2σ-(I)) 0.024 3, 0.056 7 0.028 3, 0.070 7 R1, wR2 (all data) 0.030 3, 0.075 6 0.030 4, 0.084 2 Flack 0.000(15) 0.005(13) Table 2. Selected bond lengths (nm) and bond angles (°) of 1
La (1)-N (1) 0.275 7(3) La (1)-O (2) 0.245 7(3) La (1)-O (4) 0.253 9(3) La (1)-O (4) 0.281 2(3) La (1)-O (6) 0.247 6(3) La (1)-O (7) 0.250 0(3) La (1)-O (1) 0.248 7(3) La (1)-N (3) 0.276 7(4) La (1)-C (4) 0.302 9(4) La (1)-O (3) 0.255 1(3) N (1)-La (1)-O (4) 110.65(10) N (1)-La (1)-N (3) 59.43(11) N (1)-La (1)-C (4) 91.16(11) O (2)-La (1)-N (1) 141.66(10) O (2)-La (1)-O (4) 71.26(9) O (2)-La (1)-O (4) 65.77(9) O (2)-La (1)-O (6) 74.90(10) O (2)-La (1)-O (7) 88.94(10) O (2)-La (1)-O (1) 133.84(10) O (2)-La (1)-N (3) 146.21(11) O (2)-La (1)-C (4) 71.67(10) O (2)-La (1)-O (3) 77.85(10) O (4)-La (1)-N (1) 119.20(10) O (4)-La (1)-O (4) 129.72(3) O (4)-La (1)-N (3) 74.95(10) O (4)-La (1)-C (4) 142.92(10) O (4)-La (1)-C (4) 24.64(9) O (4)-La (1)-O (3) 141.96(10) O (6)-La (1)-N (1) 72.70(10) O (6)-La (1)-O (4) 114.40(9) O (6)-La (1)-O (4) 76.51(10) O (6)-La (1)-O (7) 147.86(10) O (6)-La (1)-O (1) 137.32(10) O (6)-La (1)-N (3) 97.85(11) O (6)-La (1)-C (4) 94.86(10) O (6)-La (1)-O (3) 74.41(10) O (7)-La (1)-N (1) 129.22(10) O (7)-La (1)-O (4) 71.99(10) O (7)-La (1)-O (4) 82.18(9) O (7)-La (1)-N (3) 80.18(11) O (7)-La (1)-C (4) 106.35(10) O (7)-La (1)-O (3) 129.78(10) O (1)-La (1)-N (1) 66.90(10) O (1)-La (1)-O (4) 69.75(9) O (1)-La (1)-O (4) 135.96(10) O (1)-La (1)-O (7) 73.22(10) O (1)-La (1)-N (3) 73.24(11) O (1)-La (1)-C (4) 73.24(10) O (1)-La (1)-O (3) 81.83(10) N (3)-La (1)-O (4) 142.22(10) N (3)-La (1)-C (4) 142.08(11) O (3)-La (1)-N (1) 74.19(10) O (3)-La (1)-O (4) 48.11(9) O (3)-La (1)-N (3) 132.86(10) O (3)-La (1)-C (4) 23.55(10) Table 3. Selected bond lengths (nm) and bond angles (°) of 2
Eu (1)-O (2) 0.236 9(4) Eu (1)-O (7) 0.238 1(3) Eu (1)-O (1) 0.238 4(3) Eu (1)-O (8) 0.239 8(4) Eu (1)-O (10) 0.242 0(3) Eu (1)-O (9) 0.242 3(3) Eu (1)-N (2) 0.266 5(4) Eu (1)-N (1) 0.268 0(5) Eu (1)-O (10) 0.286 2(3) O (2)-Eu (1)-O (7) 75.72(13) O (2)-Eu (1)-O (1) 132.07(12) O (7)-Eu (1)-O (1) 137.28(14) O (2)-Eu (1)-O (8) 86.29(13) O (7)-Eu (1)-O (8) 147.85(13) O (1)-Eu (1)-O (8) 74.03(13) O (2)-Eu (1)-O (10) 71.93(12) O (7)-Eu (1)-O (10) 75.94(13) O (1)-Eu (1)-O (10) 137.13(13) O (8)-Eu (1)-O (10) 73.20(12) O (2)-Eu (1)-O (9) 77.57(13) O (7)-Eu (1)-O (9) 75.38(11) O (1)-Eu (1)-O (9) 80.32(12) O (8)-Eu (1)-O (9) 126.85(12) O (10)-Eu (1)-O (9) 142.24(12) O (2)-Eu (1)-N (2) 146.06(13) O (7)-Eu (1)-N (2) 98.23(13) O (1)-Eu (1)-N (2) 74.52(12) O (8)-Eu (1)-N (2) 81.94(15) O (10)-Eu (1)-N (2) 74.20(13) O (9)-Eu (1)-N (2) 134.03(14) O (1)-Eu (1)-N (2) 74.52(12) O (1)-Eu (1)-N (2) 74.52(12) O (1)-Eu (1)-N (2) 74.52(12) O (2)-Eu (1)-N (1) 141.14(13) O (7)-Eu (1)-N (1) 72.13(13) O (1)-Eu (1)-N (1) 67.55(12) O (8)-Eu (1)-N (1) 132.10(13) O (10)-Eu (1)-N (1) 119.25(12) O (9)-Eu (1)-N (1) 73.69(13) N (2)-Eu (1)-N (1) 61.34(14) O (2)-Eu (1)-O (10) 65.16(11) O (7)-Eu (1)-O (10) 115.27(11) O (1)-Eu (1)-O (10) 68.37(11) O (8)-Eu (1)-O (10) 79.17(12) O (10)-Eu (1)-O (10) 129.82(4) O (9)-Eu (1)-O (10) 48.02(11) N (2)-Eu (1)-O (10) 141.70(12) N (1)-Eu (1)-O (10) 110.44(12) -

 扫一扫看文章
扫一扫看文章
计量
- PDF下载量: 5
- 文章访问数: 653
- HTML全文浏览量: 90



 下载:
下载:






 下载:
下载:

